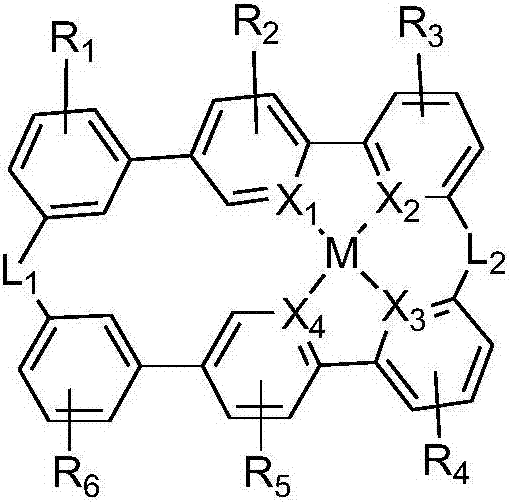
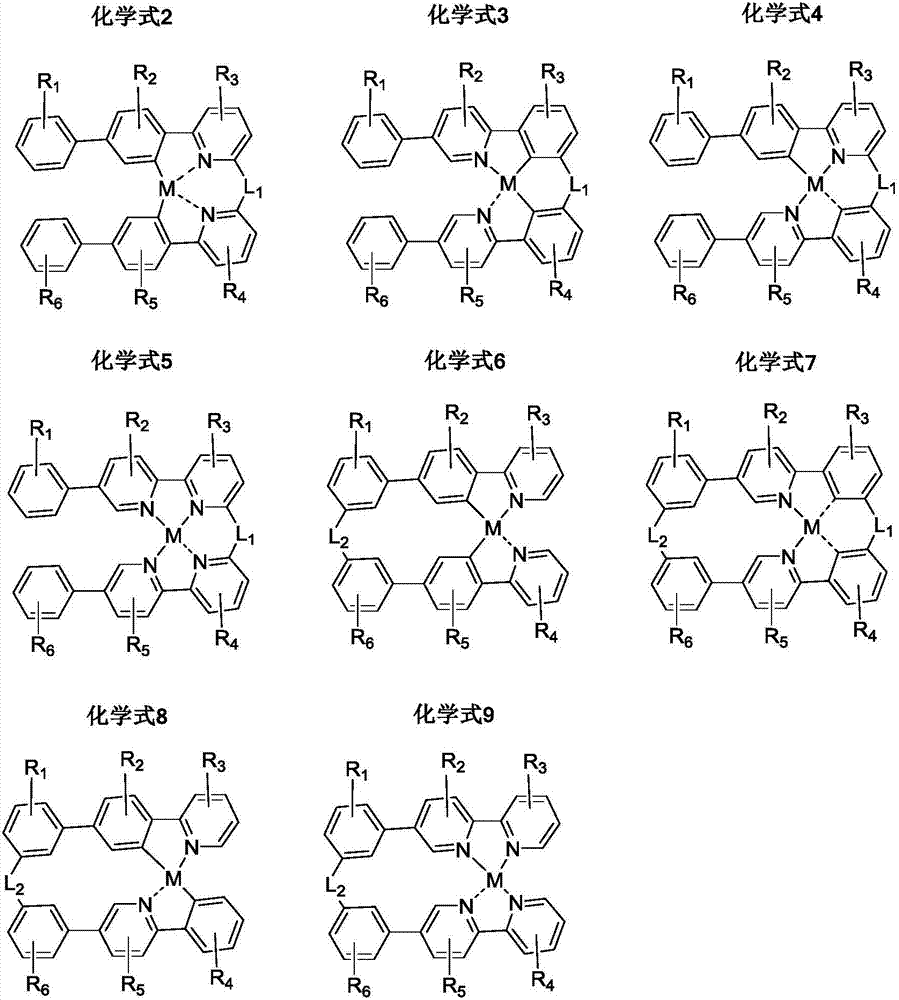
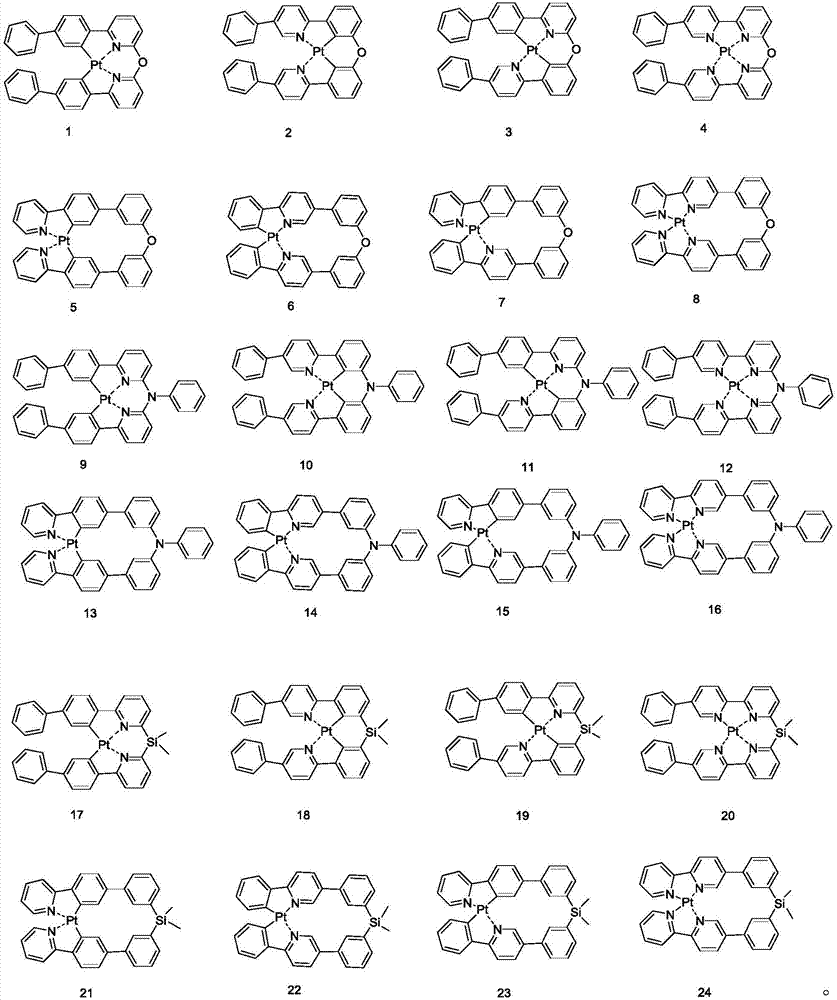
Organic metal complex, synthesis method and organic light-emitting device thereof
An organic light-emitting device, organic metal technology, applied in the direction of organic light-emitting devices, platinum group organic compounds, platinum group organic compounds, etc., can solve problems such as poor stability and low efficiency, and achieve low efficiency, good luminous efficiency and stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] [Example 1] Synthesis of compound 8
[0058]
[0059] *Synthesis of Intermediate 8-1
[0060]Add 5-bromo-2,2'bipyridine (1.97g, 8.4mmol), (3-bromophenyl)boronic acid (1.91g, 9.5mmol), tetrakistriphenylphosphine palladium (0.36g, 0.56mmol) into the reactor ), potassium carbonate (2.78g, 20.1mmol), ethylene glycol dimethyl ether 40mL, ethanol 15mL and distilled water 15mL, stirred and refluxed at 120°C for 12h. After the reaction was completed, the reaction was stopped with distilled water, extracted with ethyl acetate, and the organic layer was extracted with MgSO 4 After drying, the solvent was distilled off under reduced pressure, followed by separation and purification by column chromatography to obtain intermediate 10-1 (2.09 g, 80%).
[0061] *Synthesis of Intermediate 8-2
[0062] Add intermediate 8-1 (3.11g, 10mmol), CuI (0.19g, 1mmol), KOH (3.37g, 60mmol), 24mL DMSO / 6mL water mixed solution into the reactor, under nitrogen protection, at 120°C Stir and ref...
Embodiment 2
[0067] [Example 2] Synthesis of compound 16
[0068]
[0069] *Synthesis of intermediate 16-1
[0070] Under nitrogen protection, palladium acetate (67.35mg, 0.3mmol), tri-tert-butylphosphine (0.243g, 1.2mmol), potassium tert-butoxide (5.39g, 48mmol), intermediate 8-1 (6.22g , 20mmol), arylamine (1.02g, 11mmol), 60mL toluene, stirred and refluxed at 130°C for 10h, cooled the reactant to room temperature, diluted with toluene, filtered through diatomaceous earth, diluted the filtrate with water, and extracted with toluene , the combined organic phases were evaporated in vacuo, and the residue was separated and purified by column chromatography to obtain intermediate 16-1 (4.54 g, 82%).
[0071] *Synthesis of Compound 16
[0072] Potassium tetrachloroplatinate (2.29g, 5.5mmol), 16-1 (2.77g, 5mmol), and 300mL ethylene glycol monoethyl ether / 100mL water mixed solution were added to the reaction vessel, and magnetically stirred at 100°C for 10h under nitrogen protection. Afte...
Embodiment 3
[0073] [Example 3] Synthesis of compound 24
[0074]
[0075] *Synthesis of intermediate 24-1
[0076] Under the protection of nitrogen, THF 60mL and intermediate 8-1 (6.22g, 20mmol) were added to the reactor. At -78°C, n-Buli (1.28g, 20mmol) was added dropwise, stirred for 0.5h, and then added with di Methyldichlorosilane (1.48g, 11.5mmol), reacted at -78°C for 18h, then raised the temperature to room temperature, diluted with distilled water after the reaction, stopped the reaction, distilled the organic layer to remove the solvent under reduced pressure, and purified it with a silica gel column Intermediate 24-1 (8.12 g, 78%) was obtained.
[0077] *Synthesis of Compound 24
[0078] Potassium tetrachloroplatinate (2.29g, 5.5mmol), 24-1 (2.6g, 5mmol), and 300mL ethylene glycol monoethyl ether / 100mL water mixed solution were added to the reaction vessel, and magnetically stirred at 100°C for 10h under nitrogen protection. After the reaction was completed, suction filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com