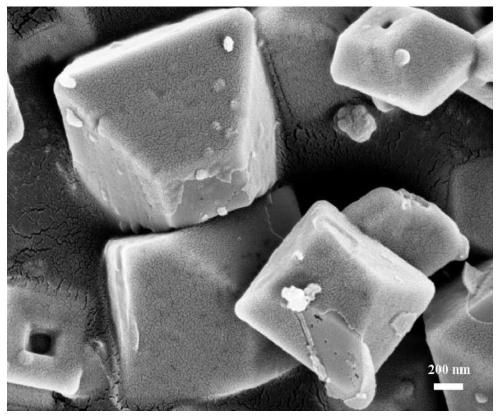
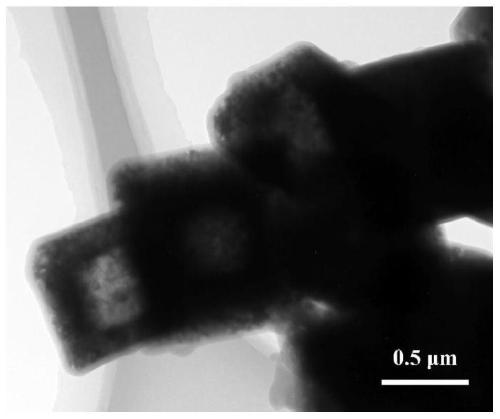
Preparation method of carbon-coated manganese tetraoxide polyhedron negative electrode material for lithium ion battery
A technology of carbon-coated manganese tetroxide and lithium ion batteries, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of reduced electrode cycle life, reduced electrochemical performance, large irreversible capacity, etc., to avoid capacity decay The effect of too fast, improving structural stability, and improving electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) According to m 甲酰胺 :m 高锰酸钾 =48, fully stirring and dissolving potassium permanganate in formamide;
[0034] (2) According to m 水 :m 聚乙二醇 =15.8, polyethylene glycol 20000 was dissolved in deionized water to obtain a uniform mixed solution;
[0035] (3) According to m 步骤(2) :m 步骤(1) =1.38, the mixed solution obtained in step (2) was added to the mixed solution in step (1), and stirred at 80° C. for 15 min;
[0036] (4) Put the sol obtained in step (3) into a hydrothermal kettle, and react at 100° C. for 14 hours;
[0037] (5) After cooling to room temperature, the solid in the product obtained in step (4) was washed several times with deionized water and ethanol, and the washed product was placed in a vacuum oven at 50-60°C to dry;
[0038] (6) According to m 固体 :m 三羟甲基氨基甲烷 = 4.2, the solid obtained in step (5) was added to the prepared tris buffer (pH=8.5) with a molar concentration of 10 mM, and ultrasonicated for 0.5 h;
[0039] (7) According to the mass ...
Embodiment 2
[0044] (1) According to m 甲酰胺 :m 高锰酸钾 =47, fully stirring and dissolving potassium permanganate in formamide;
[0045] (2) According to m 水 :m 聚乙二醇 =14.5, polyethylene glycol 20000 was dissolved in deionized water to obtain a uniform mixed solution;
[0046] (3) According to m 步骤(2) :m 步骤(1) =1.32, the mixed solution obtained in step (2) was added to the mixed solution in step (1), and stirred at 80° C. for 15 min;
[0047] (4) Put the sol obtained in step (3) into a hydrothermal kettle, and react at 120° C. for 14 hours;
[0048] (5) After cooling to room temperature, the solid in the product obtained in step (4) was washed several times with deionized water and ethanol, and the washed product was placed in a vacuum oven at 50-60°C to dry;
[0049] (6) According to m 固体 :m 三羟甲基氨基甲烷 = 4.0, the solid obtained in step (5) was added to the prepared tris buffer solution (pH = 8.5) with a molar concentration of 10 mM, and ultrasonicated for 0.5 h;
[0050] (7) According t...
Embodiment 3
[0055] (1) According to m 甲酰胺 :m 高锰酸钾 =46, fully stirring and dissolving potassium permanganate in formamide;
[0056] (2) According to m 水 :m 聚乙二醇 =14.7, polyethylene glycol 20000 was dissolved in deionized water to obtain a uniform mixed solution;
[0057] (3) According to m 步骤(2) :m 步骤(1) =1.41, the mixed solution obtained in step (2) was added to the mixed solution in step (1), and stirred at 80° C. for 15 min;
[0058] (4) Put the sol obtained in step (3) into a hydrothermal kettle, and react at 140° C. for 14 hours;
[0059] (5) After cooling to room temperature, the solid in the product obtained in step (4) was washed several times with deionized water and ethanol, and the washed product was placed in a vacuum oven at 50-60°C to dry;
[0060] (6) According to m 固体 :m 三羟甲基氨基甲烷 = 3.9, the solid obtained in step (5) was added to the prepared tris buffer solution (pH = 8.5) with a molar concentration of 10 mM, and ultrasonicated for 0.5 h;
[0061] (7) According t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com