Cultivation system for in-vitro amplification of lymphocytes and amplification method and application
A culture system and lymphocyte technology, applied in cell culture active agents, cells modified by introducing foreign genetic material, animal cells, etc., can solve the problem of difficulty in obtaining a sufficient number of NK cells, and achieve uniform quality, strong lethality, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 genetic engineering cell
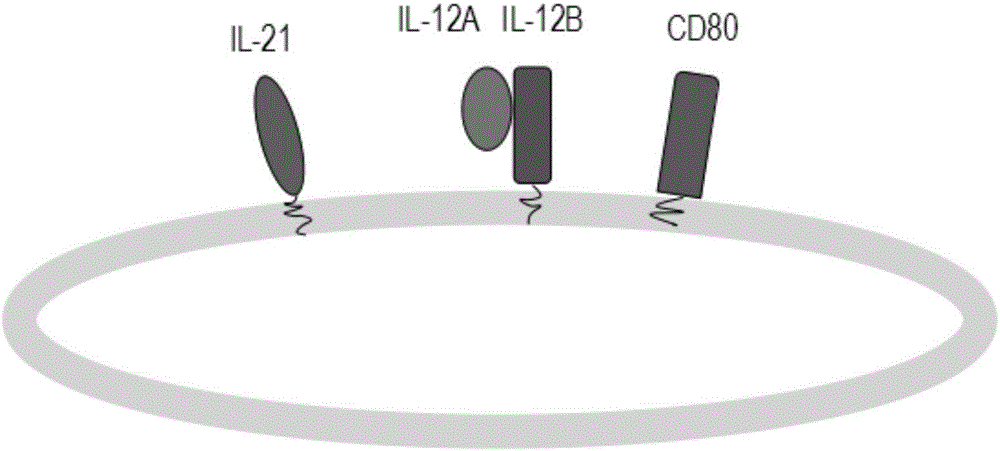
[0057] The preparation method of the genetically engineered cells is as follows: Firstly, a vector capable of stably expressing transmembrane interleukin 21, interleukin IL12A, transmembrane interleukin 12B and CD80 was constructed. There is a selectable marker gene on the vector. Transmembrane interleukin 21 or transmembrane interleukin 12B is connected to the cell membrane through the transmembrane part of CD4, and interleukin IL12A and CD80 are transmembrane proteins. These vectors were transfected into K562 cells, and high-expressing cell clones were selected with antibiotics and flow cytometry.
Embodiment 2
[0058] The preparation of embodiment 2 lymphocyte expansion culture system
[0059] The genetically engineered cells prepared in Example 1 are used for the expansion of lymphocytes, comprising the following steps:
[0060] (1) Inactivated genetically engineered cells: irradiate with 100Gy radiation for 30 minutes to obtain inactivated K562 engineered cells;
[0061] (2) Preparation of culture medium: take lymphocyte culture medium RPMI1640 and 10% fetal bovine serum, then add the inactivated K562 engineered cells of step (1) to the mixed medium, and the addition amount is 1×10 6 / mL, then add IL-2, the amount of IL-2 added is 50U / mL.
Embodiment 3
[0062] Example 3 Expansion of Lymphocytes from Healthy Volunteers
[0063] (1) Culture: Collect blood from fresh healthy volunteers, collect serum by centrifugation for storage, and obtain human peripheral blood mononuclear cells (PBMC) through lymph separation fluid separation, and the inoculation density is 1×10 6 / mL (according to the ratio of the K562 engineered cells to the monocytes is 1:1), inoculated into the culture system of in vitro amplified lymphocytes;
[0064] (2) Amplification: 37°C, 5% CO 2 Cultivate under conditions for 7 days, then add the above culture system, and cultivate for another 7 days;
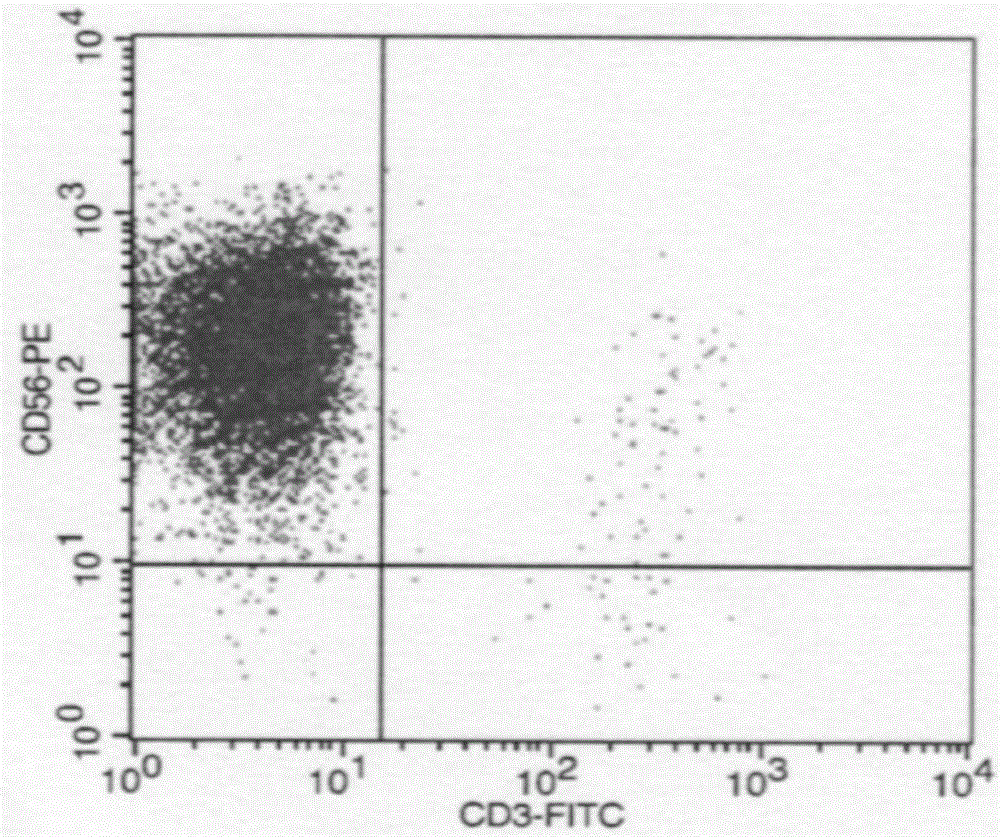
[0065] (3) Harvesting: NK cells were collected by centrifugation after the culture was completed.
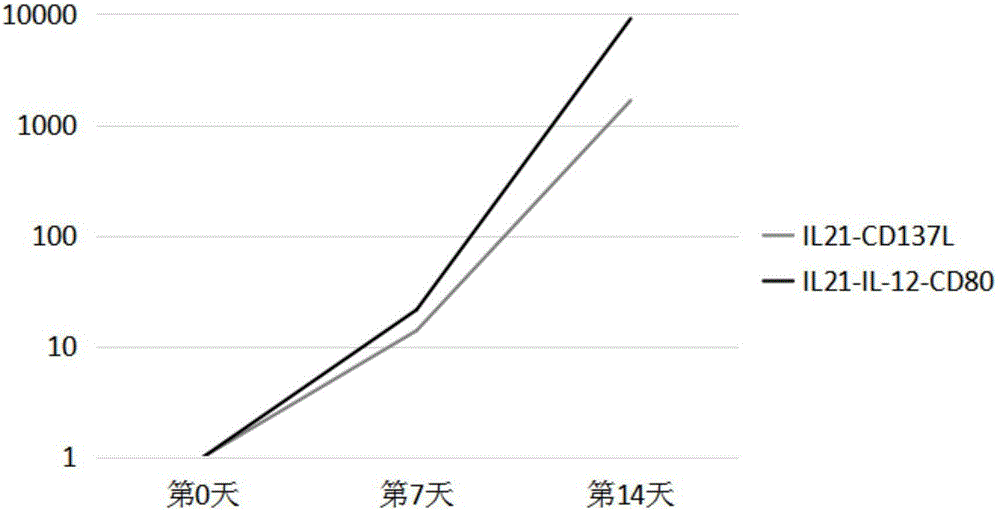
[0066] NK cells co-cultured with PBMC cells from K562 cells transfected with transmembrane interleukin 21 and CD137L were used as controls;
[0067] Use an automatic cell counter to count the expanded NK cells, and draw a curve, the growth curve is as follows fig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inoculation density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com