IL-17 antibody
A technology for IL-17 and antibodies, applied in the direction of antibodies, anti-inflammatory agents, anti-tumor drugs, etc. Refrigeration and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1: Antibody Preparation
[0102] 1. Carrier Construction
[0103] First synthesize the coding genes of the heavy chain H3-1 of the antibody and the light chain sequence L3, after PCR amplification, the DNA sequence and the pcdna3.1 vector (Invitrogen, V79020) was subjected to double enzyme digestion, and the digested vector and target DNA fragments were recovered separately, and then ligated using a DNA ligase kit (Takara / 6022) at 16°C for 1 hour to ligate the heavy chain and the digested product of the vector, the light chain and the vector, respectively. The product was digested, so that the heavy chain and light chain of the antibody were respectively constructed on the pcdna3.1 vector to form the heavy chain pcdna3.1 (H3-1) and light chain plasmid pcdna3.1 (L3).
[0104] The above-mentioned heavy chain H3-1 coding gene includes a signal peptide sequence (ATGGGATGGTCATGTATCATCCTTTTTCTAGTAGCAACTGCCACCGGTGTACACTCA, SEQ ID NO: 35), a heavy chain variable region...
Embodiment 2
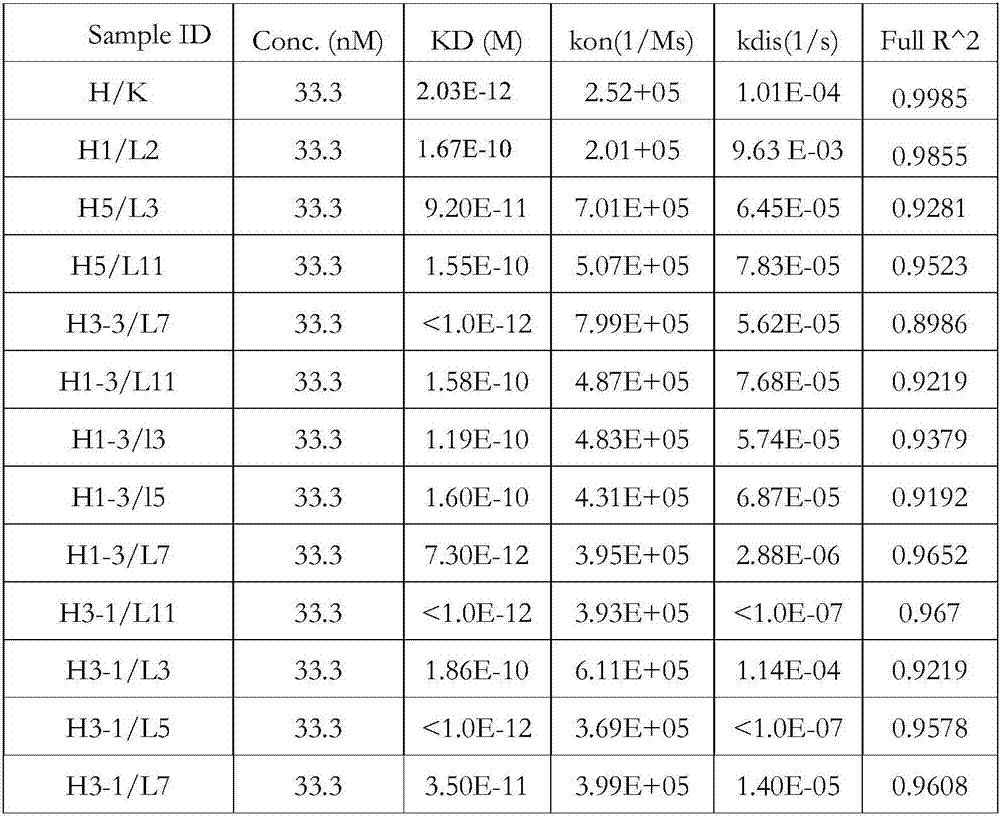
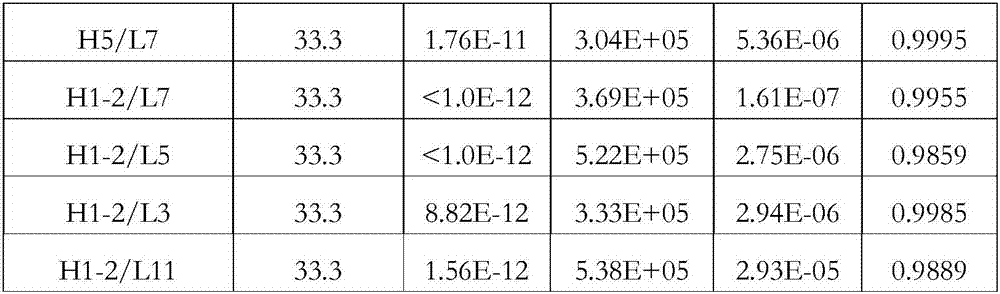
[0123] Example 2 KD Detection of Binding Affinity Between IL-17 Antibody and Antigen
[0124] 1. Instrument and principle
[0125] In this experiment, biofilm layer interferometry (BLI) was used to detect and evaluate the affinity between IL-17 antibody and antigen, so as to determine the superiority of the selected antibody in terms of kinetics and affinity. The experiment was carried out using an Octet instrument (Fortebio company, model QKe). Biotinylation kit (EZ-Link TM Sulfo-NHS-Biotin cat:21326) for biotin labeling.
[0126] 2. Experimental method
[0127] The experiment mainly includes the following steps (the experiment is carried out at room temperature unless otherwise specified): turn on the power of the machine Octet, and preheat the machine for more than 1 hour. Place the unused SA sensor on the removal rack, add 300ul DPBS buffer (0.05% BSA, 0.1% Tween) to the corresponding well below the removal rack, and incubate the sensor in the buffer for more than 10mi...
Embodiment 3
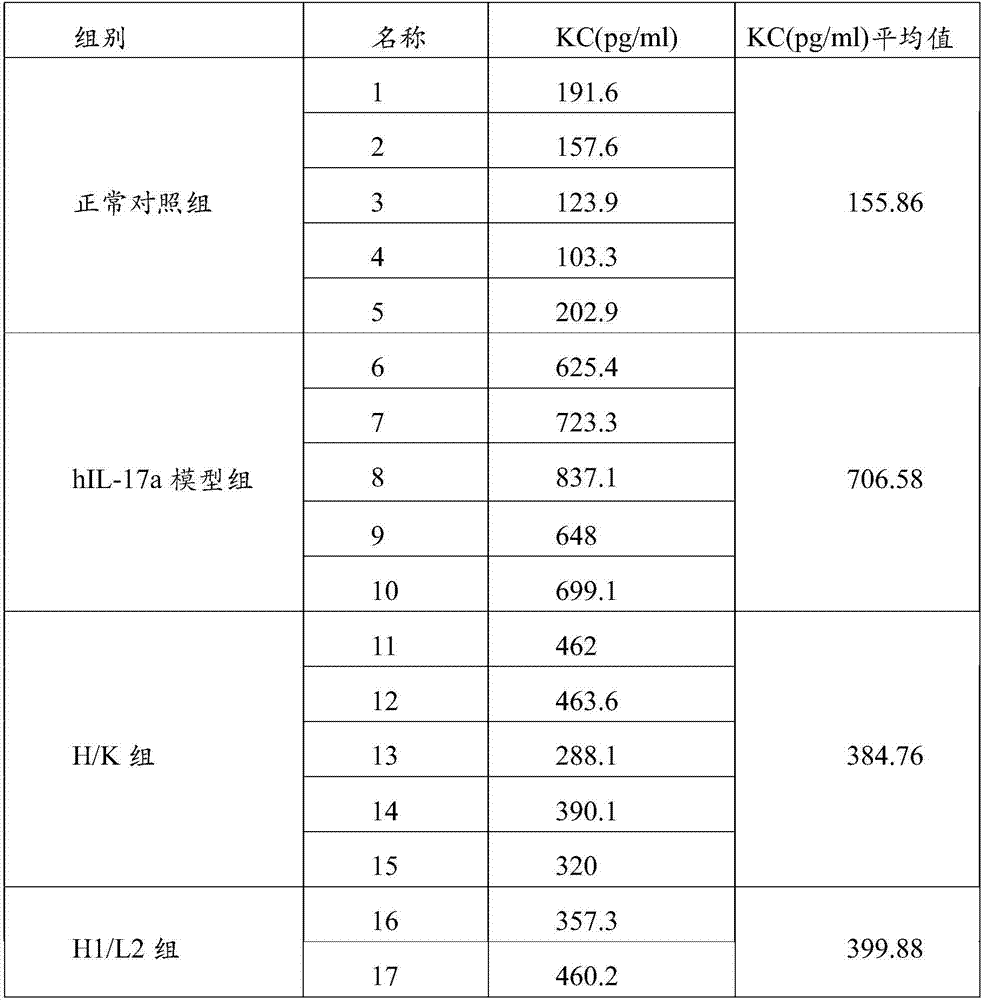
[0146] Example 3 Determination of hIL-17 Antibody Inhibitory Activity
[0147] 1. Principles and materials
[0148] The hIL-17 antibody was used to act on hIL-17A-stimulated human embryonic skin fibroblasts (referred to as CCC-ESF-1 cells, Concorde Cell Resource Center), and the IL- 6 level, indicating the inhibitory activity of hIL-17 antibody, in which IL-6 was quantified with human IL-6 Elisa detection kit (R&D system, D6050).
[0149] 2. Experimental method
[0150] CCC-ESF-1 cells at 1×10 4 Cells / well were seeded in 96-well cell culture plates at 37°C, 5% CO 2 Incubate overnight. Then divide into blank control group, control group and experimental group to treat the cells.
[0151] (1) The blank control group was diluted by adding DMEM cell culture medium (Gibco, 11995-065);
[0152] (2) Add 30ng / ml (1.67pmol / ml) hIL-17A (Genscript, Z03228-50) to the control group;
[0153] (3) Experimental group: when the dose of added hIL-17 antigen is constant (final concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com