Method for constructing recombinant escherichia coli and method for producing beta-alanine by fermentation
A kind of technology of recombinant Escherichia coli, construction method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 knockout of β-alanine competitive metabolic pathway
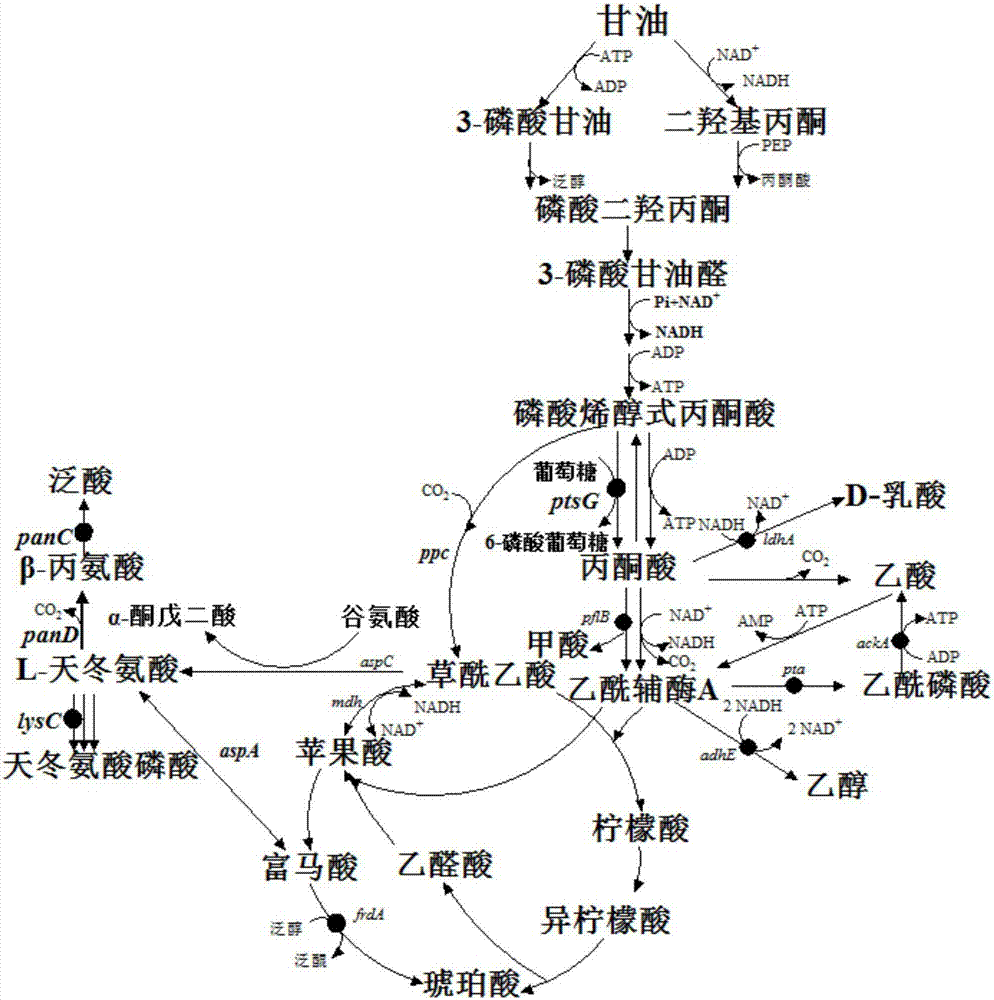
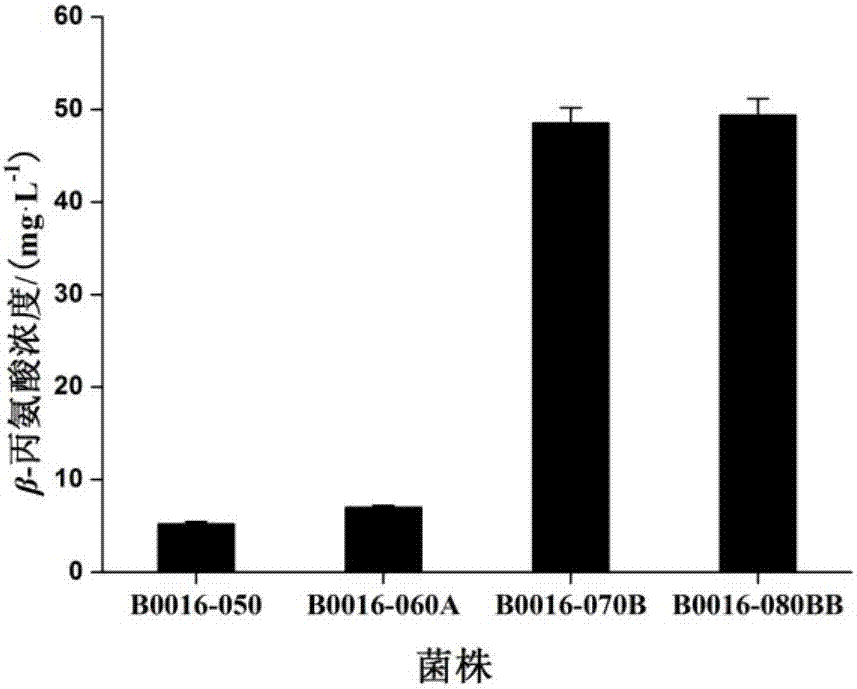
[0061] Using the method reported in the literature "Datsenko K A, et al. Proc Natl Acad Sci USA, 2000, 97: 6640-6645", the aspartokinase coding gene lysC was sequentially knocked out in the strain E.coli B0016-050 , the coding gene panC of pantothenate synthase and the coding gene ptsG of the glucose transporter EⅡCBGlc (such as figure 1 shown), B0016-060A, B0016-070B and B0016-080BB strains were obtained in sequence (see Table 1). figure 1 Among them, pflB stands for pyruvate formate lyase, ldhA stands for NAD-dependent fermentative D-lactate dehydrogenase, pta stands for phosphotransacetylase, ackA stands for acetate kinase, adhE stands for alcohol dehydrogenase, frdA stands for fumarate reductase, aspA stands for aspartate ammonia lyase, lysC stands for aspartate kinase, panC stands for pantothenate synthase, ptsG stands for glucose transporter IICB Glc, ppc stands for phosphoenolpyruvate carboxylase, a...
Embodiment 2
[0063] The heterologous overexpression of embodiment 2panD gene
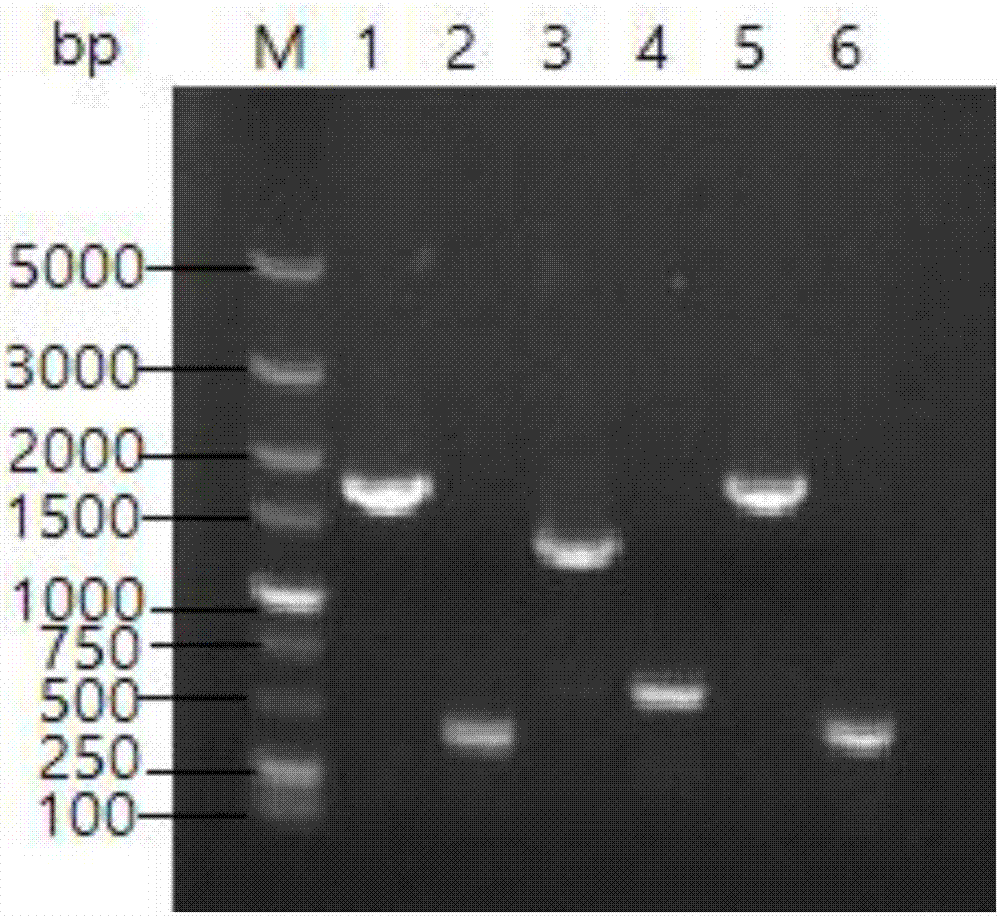
[0064] The plasmid pET28a-panD in Example 1 was double-digested with BglII and EcoR I, and the 0.5kb fragment was recovered from the gel, and cloned in the BamH I and EcoR I restriction sites of the pPL451 plasmid vector to obtain the recombinant plasmid pPL-panD (its Physical map such as Figure 4 shown in A). The recombinant plasmid pPL-panD was digested with EcoR I, and a 4.8kb band was obtained by electrophoresis ( Figure 4 B), Figure 4 M in B stands for DL 10000 DNA marker, 1 stands for pPL-panD EcoRI digestion product, Figure 4 The results showed that the recombinant plasmid pPL-panD was constructed correctly.
[0065] The pPL-panD plasmid was transformed into strains B0016-050, B0016-060A, B0016-070B and B0016-080BB, respectively, to obtain B0016-050 / pPL-panD, B0016-060A / pPL-panD, B0016-070B / pPL-panD and
[0066] B0016-080BB / pPL-panD recombinant Escherichia coli, the strains obtained above were ...
Embodiment 3
[0075] Embodiment 3 Shake flask fermentation 080BB / pPL-panD recombinant Escherichia coli
[0076] Shake flask fermentation was carried out on the strain B0016-080BB / pPL-panD obtained in Example 2, and the transition timing of the two stages of bacterial growth at 33°C and β-alanine synthesis at 42°C was optimized. When the bacterial cell growth at 33°C to OD 600 At 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, the fermentation temperature was switched from 33°C to 42°C, and the amount of β-alanine synthesis was measured.
[0077] In addition, under the optimal induction opportunity, the strain B0016-080BB / pPL-panD obtained in Example 2 was subjected to shake-flask fermentation, and the β-alanine fermentation cycle of the bacterial strain B0016-080BB / pPL-panD was further optimized. After the temperature was adjusted to 42°C, the total fermentation time was 30-65 hours, and samples were taken every 6 hours to measure the amount of β-alanine synthesized.
[0078] The optimization results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com