Azathioprine dry suspension and preparation method thereof
A technology of azathioprine and dry suspension is applied in the directions of anti-inflammatory agents, non-central pain relievers, extracellular fluid diseases, etc. Good effect, the effect of improving drug absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
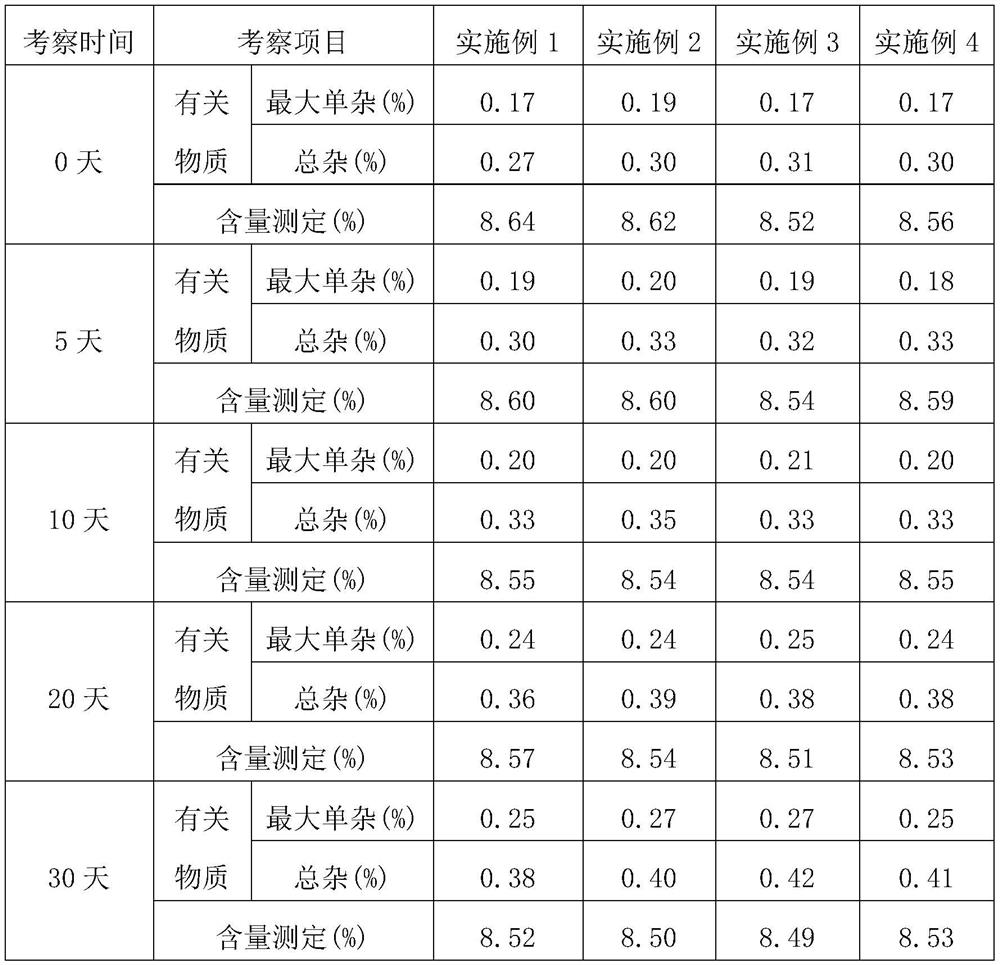
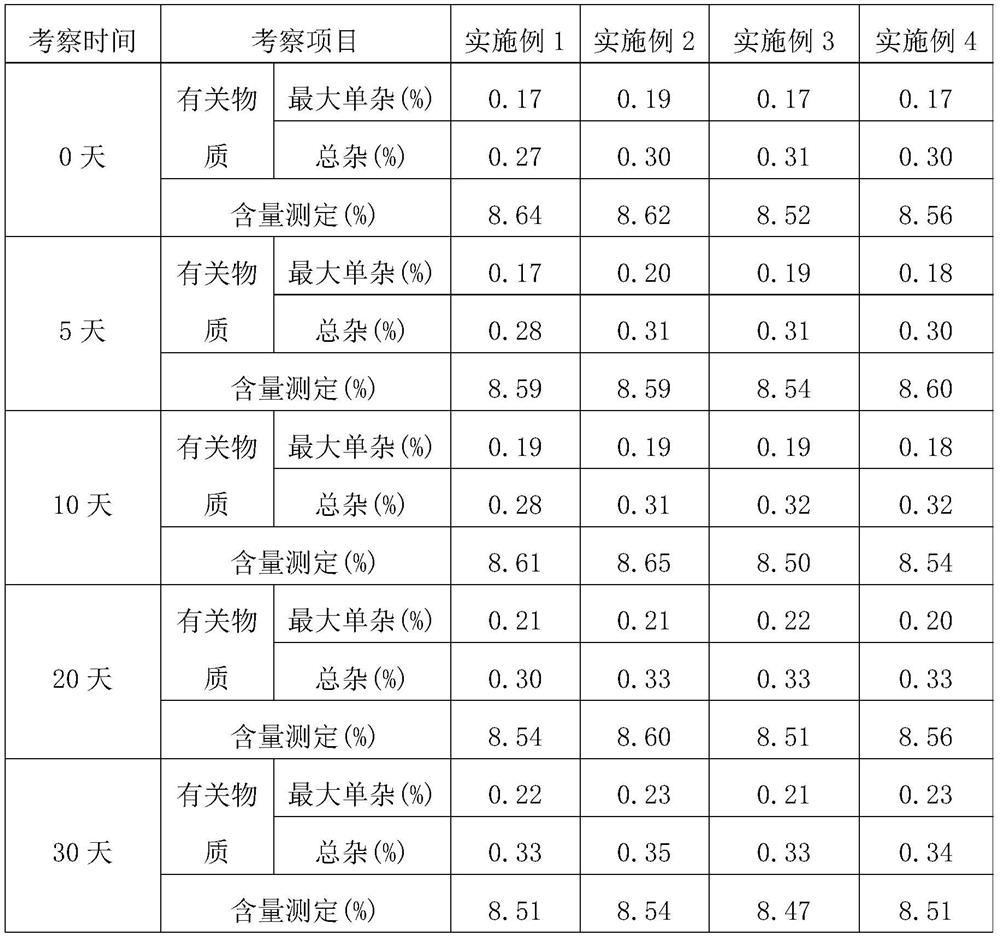
Examples
Embodiment 1
[0058] In the azathioprine dry suspension provided in this embodiment, the amounts of each raw material are as follows:
[0059] Azathioprine 50g, sorbitol 400g, polyvinylpyrrolidone 75g, micronized silica gel 3g, cross-linked polyvinylpyrrolidone 50g, 30% ethanol aqueous solution 200g, and aspartame 0.5g.
[0060] The preparation method of azathioprine dry suspension described in this embodiment includes the following steps:
[0061] 1) Take 50 g of azathioprine and 120 g of sorbitol for co-pulverization, and pass through an 80-mesh sieve after pulverization to obtain powder A;
[0062] 2) Take the remaining 280g of sorbitol, 50g of cross-linked polyvinylpyrrolidone, and 3g of micronized silica gel, all pass through a 60-mesh sieve, add powder A, mix well, add 200g of ethanol with a volume fraction of 30% in water to make a soft material;
[0063] 3) The soft material is passed through a 40-mesh sieve to form granules, dried at 60°C, and then passed through a 30-mesh sieve to obtain w...
Embodiment 2
[0066] In the azathioprine dry suspension provided in this embodiment, the amounts of each raw material are as follows:
[0067] Azathioprine 50g, sorbitol 400g, suspending agent 72g, micronized silica gel 3g, cross-linked polyvinylpyrrolidone 55g, 30% ethanol aqueous solution 100g, aspartame 0.5g; wherein, the suspending agent It is a mixture of low-substituted hydroxypropyl cellulose and polyvinylpyrrolidone with a mass ratio of 1:1.2.
[0068] The preparation method of azathioprine dry suspension described in this embodiment includes the following steps:
[0069] 1) Take 50 g of azathioprine and 120 g of sorbitol for co-pulverization, and pass through an 80-mesh sieve after pulverization to obtain powder A;
[0070] 2) Take the remaining 280g of sorbitol, 55g of cross-linked polyvinylpyrrolidone, and 3g of micronized silica gel, all pass through a 60-mesh sieve, add powder A, mix well, add 100g of ethanol with a volume fraction of 30% in water to make a soft material;
[0071] 3) T...
Embodiment 3
[0074] In the azathioprine dry suspension provided in this embodiment, the amounts of each raw material are as follows:
[0075] 50 g of azathioprine, 400 g of sorbitol, 69 g of low-substituted hypromellose, 5 g of talc, 60 g of sodium carboxymethyl starch, 200 g of an aqueous ethanol solution with a volume fraction of 30%, and 0.5 g of aspartame.
[0076] The preparation method of azathioprine dry suspension described in this embodiment includes the following steps:
[0077] 1) Take 50 g of azathioprine and 120 g of sorbitol for co-pulverization, and pass through an 80-mesh sieve after pulverization to obtain powder A;
[0078] 2) Take the remaining 280g of sorbitol, 60g of sodium carboxymethyl starch, and 5g of talc, all pass through a 60-mesh sieve, add powder A, mix well, add 200g of ethanol with a volume fraction of 30% in water to make soft material;
[0079] 3) The soft material is passed through a 40-mesh sieve to form granules, dried at 60°C, and then passed through a 30-mesh ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com