Conjugate of anti-human DLL4 (Delta-Like Ligand 4) monoclonal antibody and adriamycin
A monoclonal antibody and conjugate technology, applied in the direction of anti-tumor drugs, drug combinations, medical preparations containing active ingredients, etc., to achieve the effects of good solubility, controllability, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of antibody-drug conjugate MvM03 conjugated by anti-human DLL4 monoclonal antibody MMGZ01 and doxorubicin DOX: (1) Antibody part: MMGZ01 was purified by ProteinA column affinity chromatography, and the above-mentioned purified MMGZ01 was purified by PBS with pH 7.0 The antibody was replaced with the antibody solution system by agarose gel G25FF column molecular sieve chromatography; the antibody concentration was determined by the BCA method; 4-12% SDS-PAGE gel electrophoresis was used to verify that the purified product was electrophoretic pure; the reducing agent TCEP was mixed with the above antibody at 3 The molar ratio of : 1 was fully mixed, and after reacting for 1 hour at 4°C, the above mixture was desalted by agarose gel G25FF column molecular sieve chromatography with a pH 7.0 PBS solution containing 1M DTPA; (2) toxin part: take toxin DOX powder Fully dissolve in DMSO solution, take 2.8 mg of GMBS powder and dissolve in 1 mL of DMSO solution, mix t...
Embodiment 2
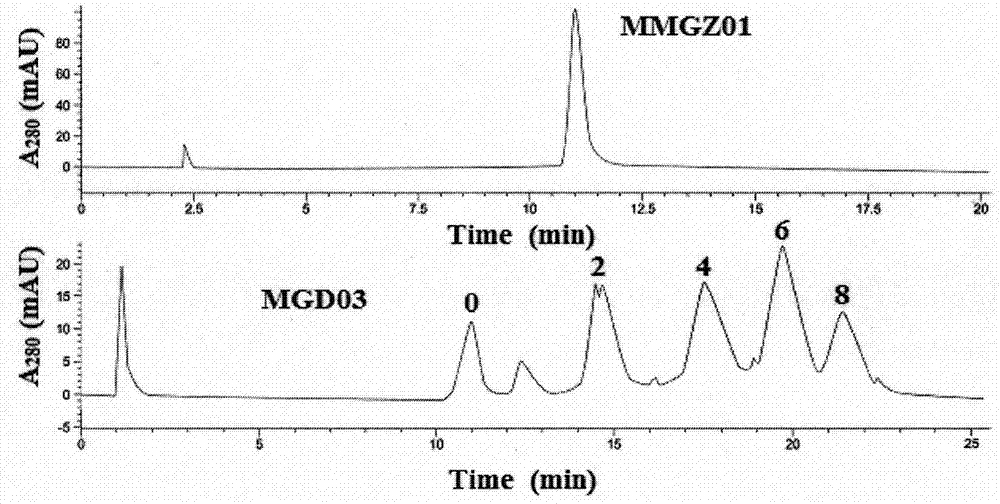
[0035] The coupling between DOX and anti-human DLL4 monoclonal antibody MMGZ01 was detected by high performance liquid chromatography (HPLC).
[0036] Agilent 1200HPLC was used to analyze the conjugation of antibody-drug conjugate MGD03. Sample detection conditions are as follows: (1) mobile phase A: 20mmol / L PBS (pH 7.0)+1.5mol / L ammonium sulfate; (2) mobile phase B: 20mmol / L PBS (pH7.0) / isopropanol=7.0 / 3.0; (3) Elution gradient: 10-100% B; (4) Elution time: 25min; (5) Flow rate: 0.60mL / min; (6) Injection volume: 10uL; (7) Detection wavelength: 280nm. According to the number of peaks and the area of each peak, the antibody-drug conjugation ratio (DAR) was calculated proportionally.
[0037] See the experimental results figure 1 , compared with MMGZ01 whose main peak appeared at 11min, MGD03 also appeared at 14.5min, 17.5min, 20min and 21.5min respectively, corresponding to the number of coupled DOX small molecules were 2, 4, 6 and 8, and the corresponding peak area pro...
Embodiment 3
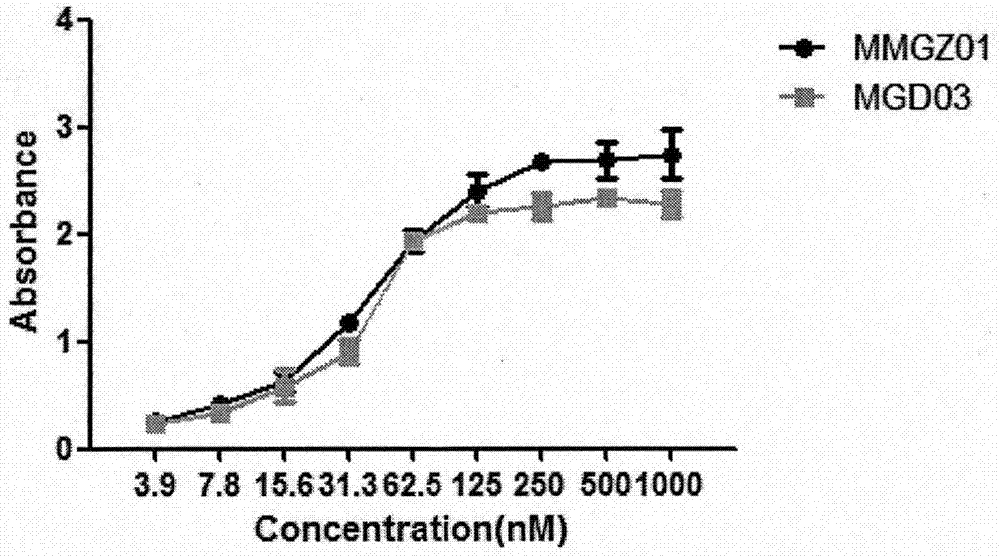
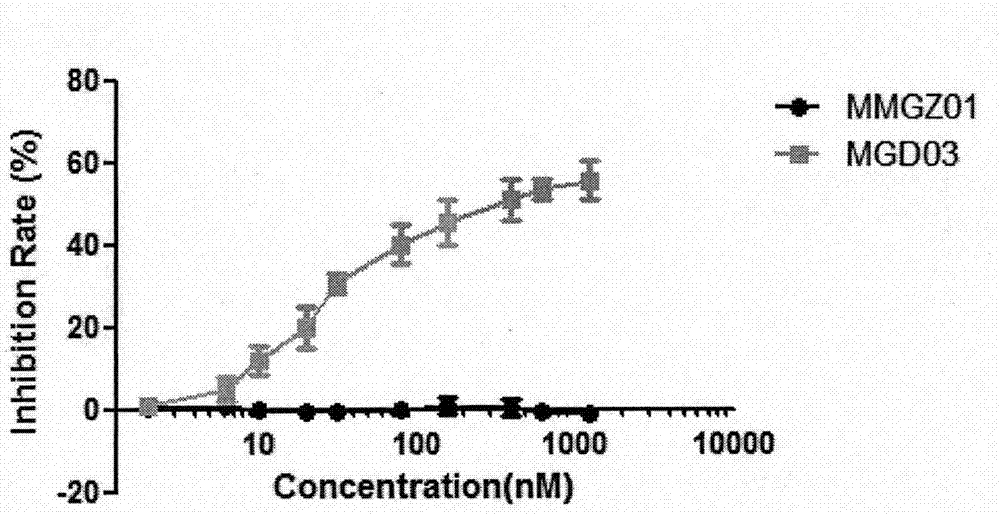
[0039] Affinity detection between antibody drug conjugate MGD03 and human DLL4 antigen: Add 1 μg / mL DLL4 antigen at 100 μL per well to a 96-well microtiter plate by ELISA method, and coat at 4°C overnight; after washing the plate three times with PBS, put The conjugates MvM03 and MMGZ01 of Example 1 were added to the 96-well plate in the blank group with concentration gradients of 0, 3.9, 7.8, 15.6, 31.3, 62.5, 125, 250, 500, and 1000 nM, and incubated at 37°C for 2 hours ; After washing the plate three times with PBS, add goat anti-mouse IgG antibody coupled with horseradish peroxidase (HRP), and incubate at 37°C for 1 hour; after washing the plate three times with PBS, add TMB chromogenic solution, React at room temperature in the dark for 20 minutes, and finally add stop solution to terminate the reaction, and detect OD450-OD630 with a microplate reader.
[0040] See the experimental results figure 2 , compared with MMGZ01, the affinity of MGD03 to DLL4 antigen is slightl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com