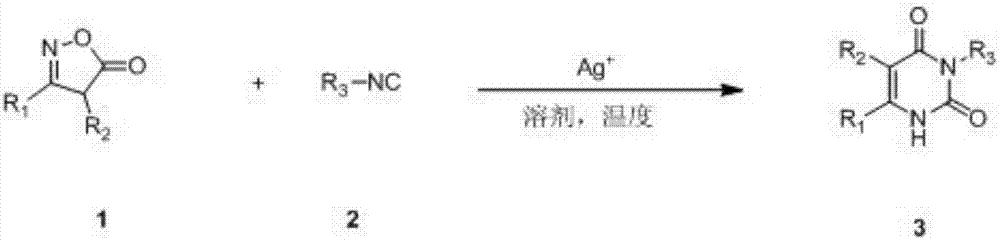
Preparation method of pyrimidine dione compounds
A technology of pyrimidine diketones and compounds, which is applied in the field of preparation of pyrimidine diketones, can solve the problems of single types of pyrimidine diketones, incapability of large-scale industrial production, and difficulty in adding functional groups, etc., to achieve superior performance and benefit The effect of large-scale industrial production and less harsh storage conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
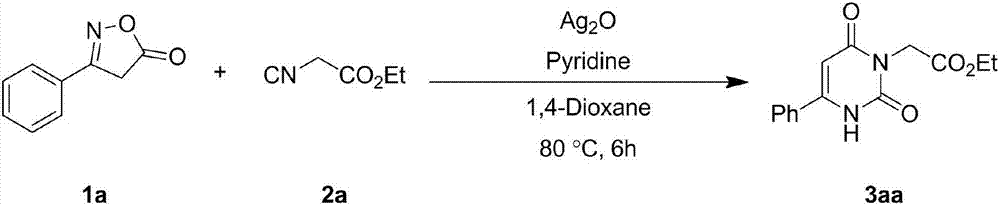
Embodiment 1
[0029]
[0030] Take a 10ml reaction tube, add 1,4-dioxane (2mL) as a solvent, weigh oxime ester compound 1a (0.2mmol), isocyanide compound 2a (0.3mmol), silver oxide (0.01mmol ), pyridine (0.2mmol), under inert gas protection, were added to the reaction tube, and the reaction tube was sealed. Stir and react in an oil bath at 80°C for 6 hours, TLC detection, after the reaction is completed, cool down to room temperature, filter with diatomaceous earth to obtain the filtrate, and rinse the filtered solid particles with ethyl acetate several times, and combine the rinses in the filtrate , and then distilled by a rotary evaporator to remove 1,4-dioxane and ethyl acetate to obtain a crude product of 3aa. The crude product of 3aa was purified by flash column chromatography to obtain a pure product of 3aa with a yield of 92%. 1 H NMR (600MHz, CDCl 3 ): δ9.97(s, 1H), 7.65(d, J=7.6Hz, 2H), 7.56(t, J=7.2Hz, 1H), 7.51(t, J=7.5Hz, 2H), 6.06(d ,J=1.4Hz,1H),4.69(s,2H),4.21(q,J=7.1Hz,2...
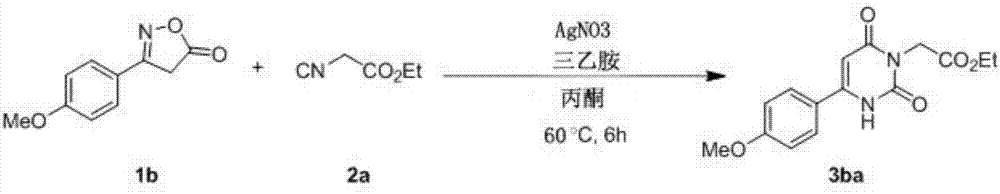
Embodiment 2
[0032]
[0033] Take a 10ml reaction tube, add acetone (2mL) as solvent, weigh oxime ester compound 1a (0.2mmol), isocyanide compound 2a (0.6mmol), AgNO 3 (0.01mmol), triethylamine (0.2mmol), under the protection of an inert gas, were added to the reaction tube, and the reaction tube was sealed. Stir and react in an oil bath at 60°C for 6 hours, TLC detection, after the reaction is completed, cool down to room temperature, filter with diatomaceous earth to obtain the filtrate, and rinse the filtered solid particles with ethyl acetate several times, and combine the rinses into the filtrate , and then distilled by a rotary evaporator to remove acetone and ethyl acetate to obtain a crude product of 3aa, and the crude product of 3aa was purified by flash column chromatography to obtain a pure product of 3aa with a yield of 79%. 1 H NMR (600MHz, CDCl 3 ):δ9.41(s,1H),7.60–7.56(m,2H),7.03–6.99(m,2H),5.99(d,J=1.5Hz,1H),4.70(s,2H),4.22( d, J=7.1Hz, 2H), 3.88(s, 3H), 1.28(t, J=7.1H...
Embodiment 3
[0035]
[0036] Take a 10ml reaction tube, add tetrahydrofuran (2mL) as a solvent, weigh oxime ester compound 1a (0.2mmol), isocyanide compound 2a (0.3mmol), AgF (0.01mmol), pyridine (0.2mmol), Under the protection of inert gas, add to the reaction tube respectively, and seal the reaction tube. Stir and react in an oil bath at 100°C for 6 hours, TLC detection, after the reaction, cool down to room temperature, filter with diatomaceous earth to obtain the filtrate, and rinse the filtered solid particles with ethyl acetate several times, and combine the rinses into the filtrate , and then distilled by a rotary evaporator to remove tetrahydrofuran and ethyl acetate to obtain a crude product of 3aa. The crude product of 3aa was purified by flash column chromatography to obtain a pure product of 3aa with a yield of 65%. 1 H NMR (600MHz, d 6 -DMSO): δ11.74(s,1H),7.93(d,J=8.2Hz,2H),7.84(d,J=8.3Hz,2H),6.10(s,1H),4.52(s,2H) ,4.11(q,J=7.1Hz,2H),1.17(t,J=7.1Hz,3H).; 13 C NMR (150MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com