Nickel boride-modified graphite-phase carbon nitride catalyst and preparation method thereof
A graphite-phase carbon nitride and nickel boride technology, applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve low conductivity, low specific surface area, few electrocatalytic fields, etc. problems, to achieve the effect of cheap raw materials, increasing the specific surface area, and increasing the pore volume of the catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Put the urea into the crucible, put the lid on and raise the temperature to 500°C in the muffle furnace at a rate of 10°C / min, keep it warm for two hours, wash and dry to obtain the graphite phase carbon nitride raw material for use. Dissolve 4.4g of nickel chloride hexahydrate evenly in 200ml of deionized water, and then add 1.41g of sodium borohydride. After the precipitation is complete, collect it by filtration, wash and dry it for later use. Mix 9.999g of graphite phase carbon nitride and 0.001g of nickel boride in an agate mortar, add 100ml of alcohol, and grind for 2 hours, during which alcohol should be continuously replenished; after drying the mixed material for 2 hours, the mixture should be dried at 5°C / min The heating rate was increased to 450°C in a muffle furnace, and kept for 2 hours. Finally, the sintered mixture was washed and dried to obtain a nickel boride-modified graphite phase carbon nitride catalyst.
[0020] Figure 4 The line scan polarization...
Embodiment 2
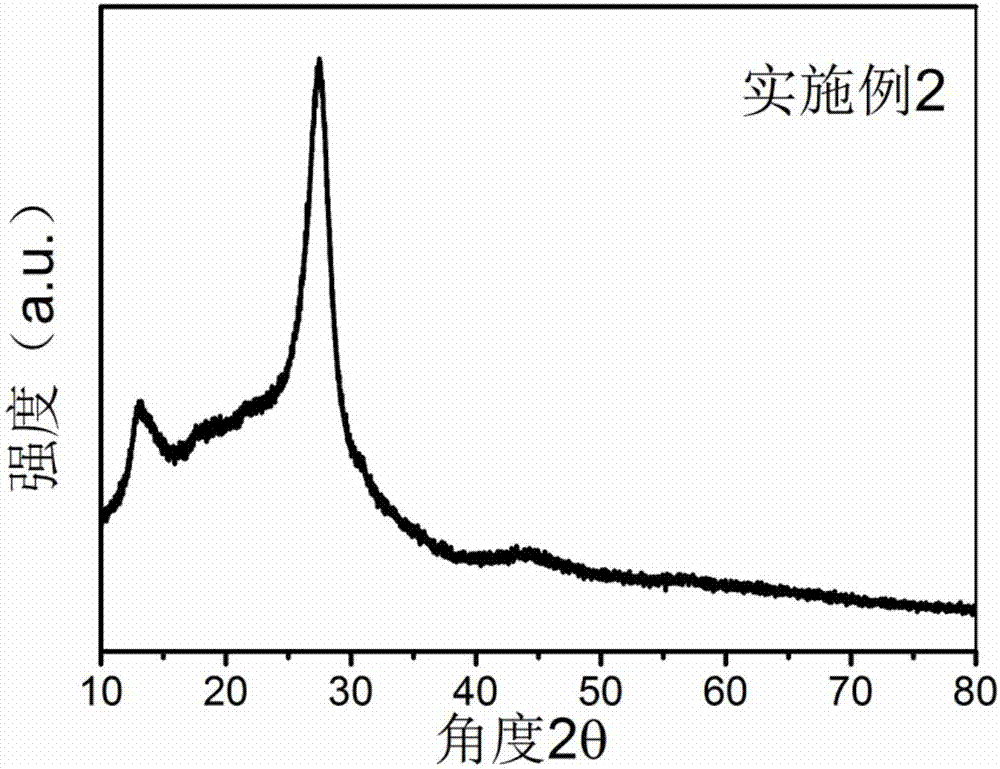
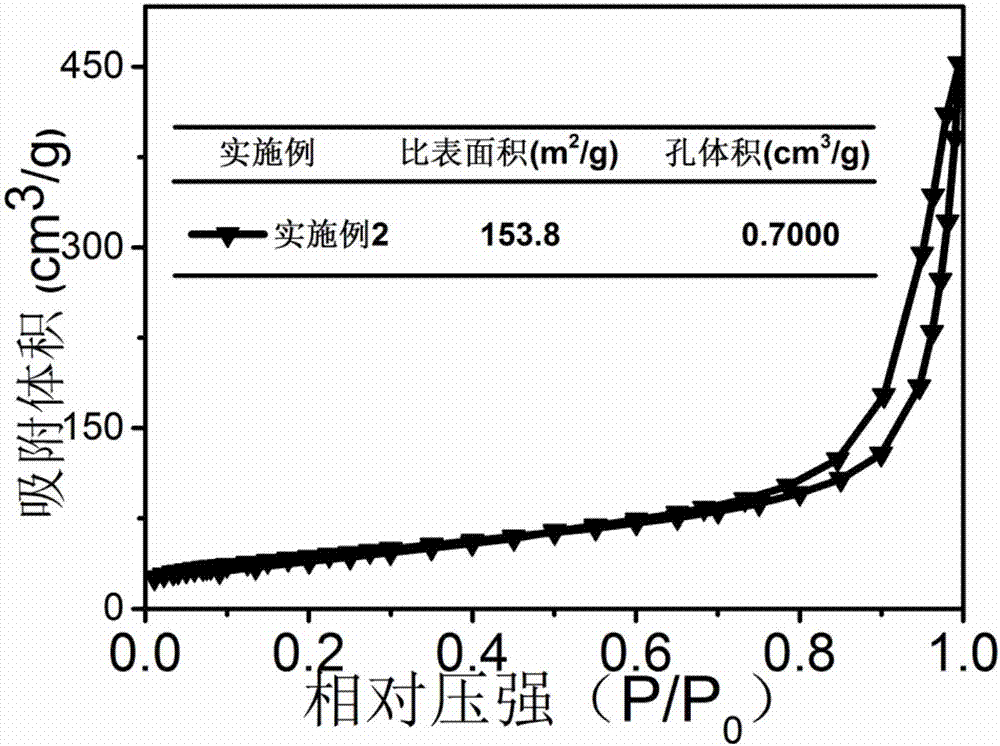
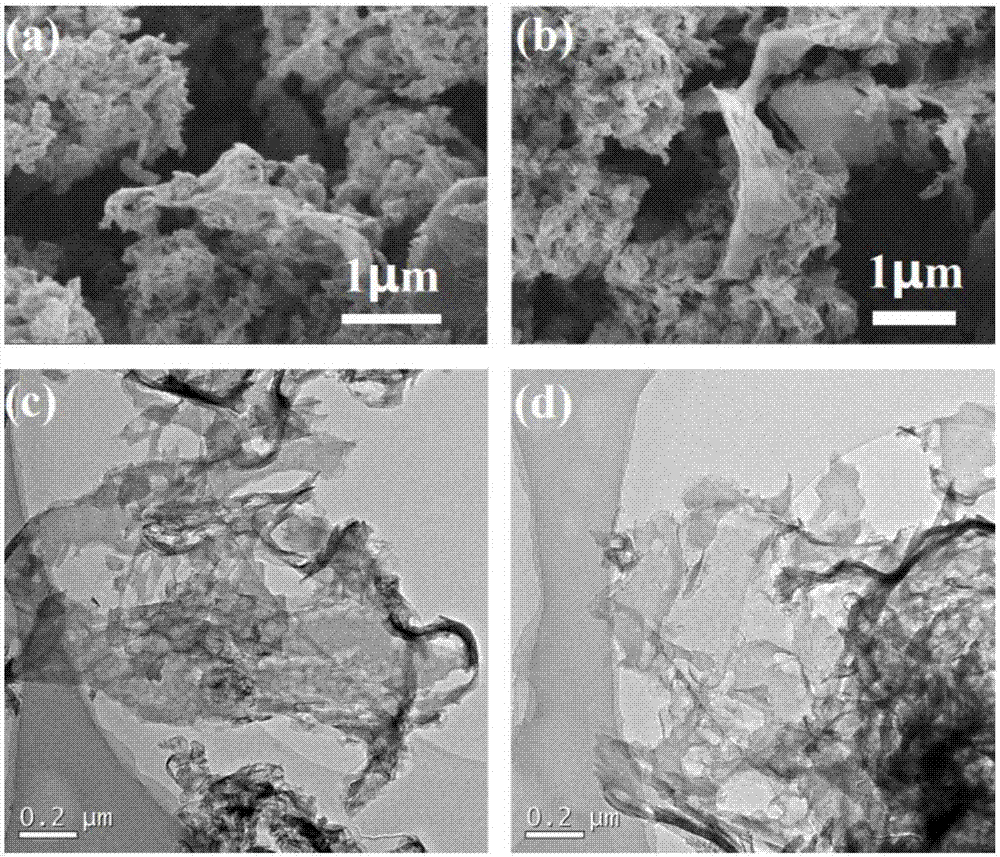
[0023] Put the urea into the crucible, put the lid on and raise the temperature to 500°C in the muffle furnace at a rate of 10°C / min, keep it warm for two hours, wash and dry to obtain the graphite phase carbon nitride raw material for use. Dissolve 4.64g of nickel chloride hexahydrate evenly in 200ml of deionized water, add 2.27g of sodium borohydride into the nickel chloride hexahydrate solution prepared above, and collect it by filtration after precipitation is complete, wash and dry for later use. Mix 9.996g of graphitic carbon nitride and 0.004g of nickel boride in an agate mortar, add 100ml of alcohol, and grind for 2 hours, during which the alcohol should be replenished continuously. After drying the mixed material for 4 hours, raise the temperature to 500°C in a muffle furnace at a rate of 10°C / min, and keep it warm for 2h, and finally wash and dry the sintered mixture to obtain nickel boride-modified graphite phase nitrogen carbonization catalyst.
[0024] Such as f...
Embodiment 3
[0030] Put the urea into the crucible, put the lid on and raise the temperature to 500°C in the muffle furnace at a rate of 10°C / min, keep it warm for two hours, wash and dry to obtain the graphite phase carbon nitride raw material for use. Dissolve 4.75g of nickel chloride hexahydrate evenly in 200ml of deionized water, add 2.65g of sodium borohydride into the nickel chloride hexahydrate solution prepared above, and wait until the precipitation is complete, collect it by filtration, wash and dry, and prepare it several times until you get Enough nickel boride. 9.993 g of graphitic carbon nitride and 0.007 g of nickel boride were mixed in a mixer for 2 hours. After drying the mixed material for 3 hours, raise the temperature to 550°C in a muffle furnace at a rate of 15°C / min, and keep it warm for 2.5h, and finally wash and dry the sintered mixture to obtain a nickel boride-modified graphite phase carbon nitride catalyst.
[0031] Figure 4 The line scan polarization curve o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com