A kind of magnesium cobaltate catalyst and its preparation method and application
A technology of magnesium cobaltate and catalyst, which is applied in the field of catalyst preparation and can solve problems not related to the application of preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 8 grams of glucose and dissolve it in 45 milliliters of deionized water to form a solution, transfer it to a self-pressurized reactor equipped with a polytetrafluoroethylene liner, raise the temperature to 180 °C at a rate of 10 °C / min, and crystallize for 6 Hour. A tan precipitate was obtained, which was washed alternately with ethanol and deionized water. Move it into an oven and dry at 80°C for 12 hours to obtain carbon spheres. The above is the process of hydrothermally synthesizing carbon spheres using glucose as a raw material, and the conditions and methods for synthesizing carbon spheres in the following examples are the same as this example.
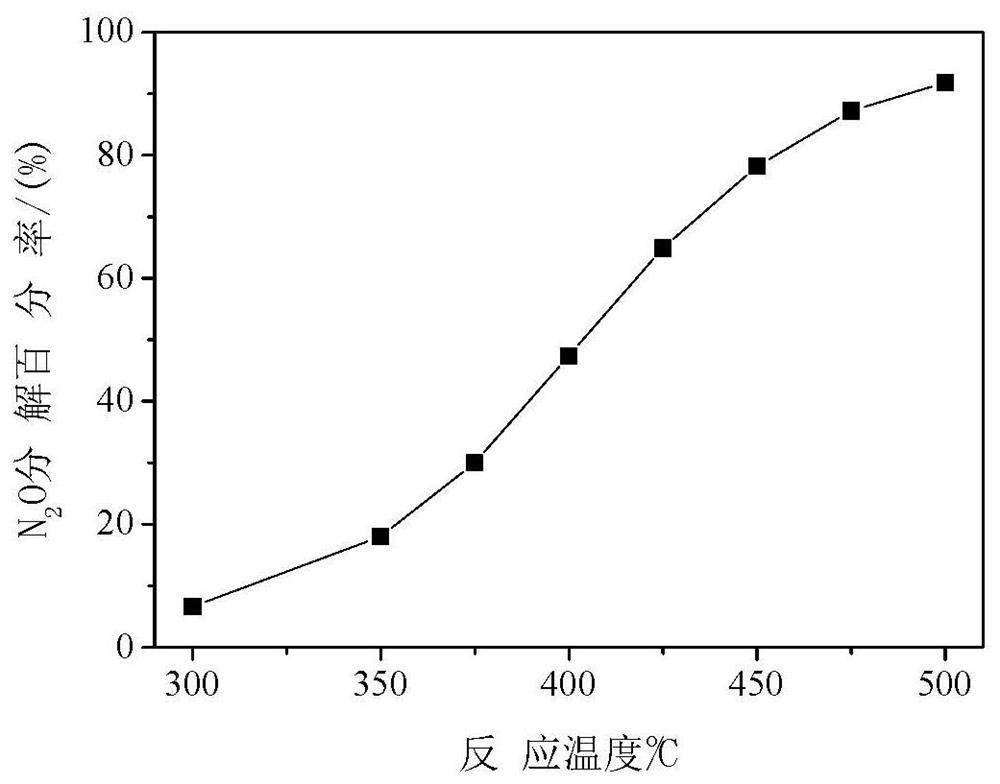
[0034] Weigh 0.611 g of Co(NO 3 ) 2 ·6H 2 O, 0.269 g Mg(NO 3 ) 2 ·6H 2 0, 0.757 gram of urea (urea molecule / cobalt-magnesium atom=4, mol ratio), be dissolved in 45 milliliters of deionized water, add in 1 gram of carbon spheres (cobalt-magnesium atom / carbon sphere=0.149, mass ratio), stir, Sonicate for 10 mi...
Embodiment 2
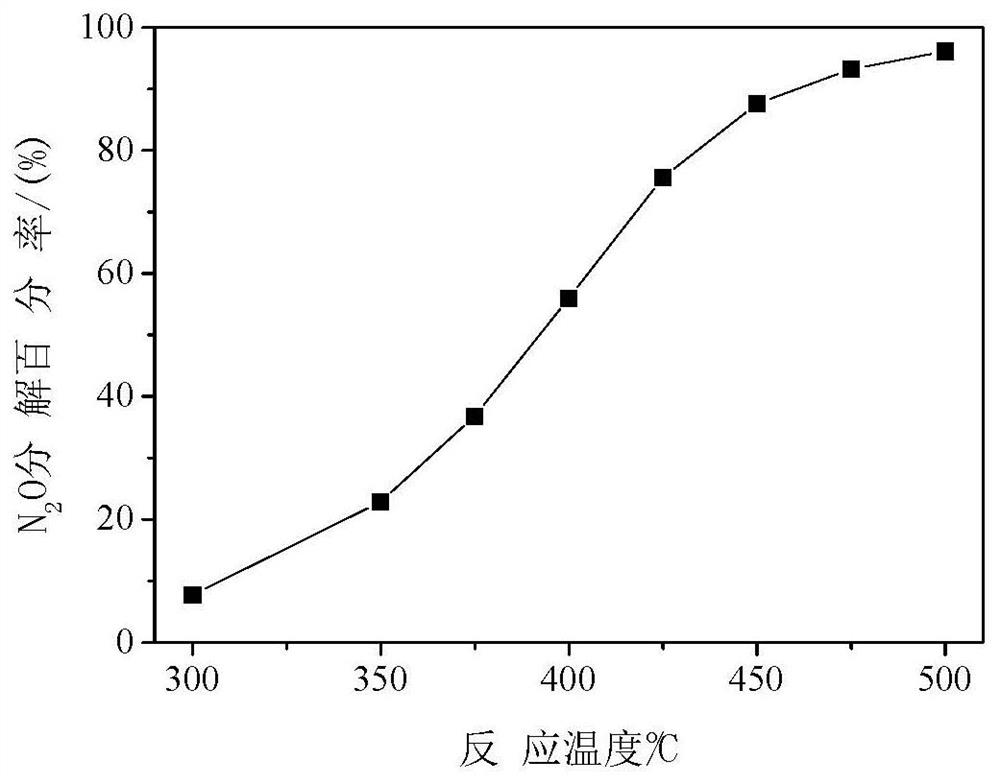
[0037] Weigh 0.786 g of Co(NO 3 ) 2 ·6H 2 O, 0.346 g Mg(NO 3 ) 2 ·6H 2 0, 0.973 gram of urea (urea molecule / cobalt-magnesium atom=4, mol ratio), be dissolved in 45 milliliters of deionized water, add in 1 gram of carbon spheres (cobalt-magnesium atom / carbon sphere=0.192, mass ratio), stir, Sonicate for 10 minutes, transfer to a self-pressurized reactor equipped with a polytetrafluoroethylene liner, seal it, raise the temperature to 120°C at a rate of 10°C / min, rotate and crystallize for 4 hours, and wash the product several times with deionized water , dried at 80°C for 12 hours. In the air, the temperature was raised to 500° C. at a rate of 5° C. / minute, and the above product was roasted at a constant temperature for 4 hours to prepare a magnesium cobaltate catalyst. For catalytic decomposition of N 2 O, N 2 See the appendix for the decomposition percentage data of O figure 2 .
Embodiment 3
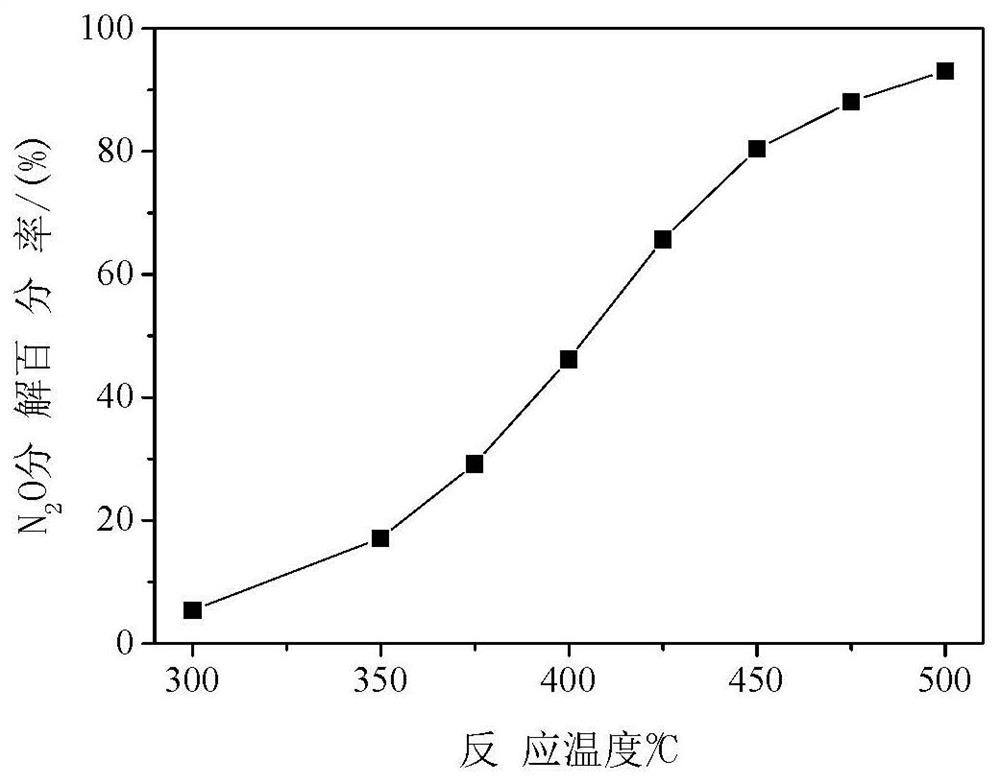
[0039] Weigh 0.960 g of Co(NO 3 ) 2 ·6H 2 O, 0.423 g Mg(NO 3 ) 2 ·6H 2 0, 1.189 grams of urea (urea molecule / cobalt-magnesium atom=4, mol ratio), be dissolved in 45 milliliters of deionized water, add in 1 gram of carbon spheres (cobalt-magnesium atom / carbon sphere=0.235, mass ratio), stir, Sonicate for 10 minutes, transfer to a self-pressurized reactor equipped with a polytetrafluoroethylene liner, seal it, raise the temperature to 120°C at a rate of 10°C / min, rotate and crystallize for 4 hours, and wash the product several times with deionized water , dried at 80°C for 12 hours. In the air, the temperature was raised to 500° C. at a rate of 5° C. / minute, and the above product was roasted at a constant temperature for 4 hours to prepare a magnesium cobaltate catalyst. For catalytic decomposition of N 2 O, N 2 See the appendix for the decomposition percentage data of O image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com