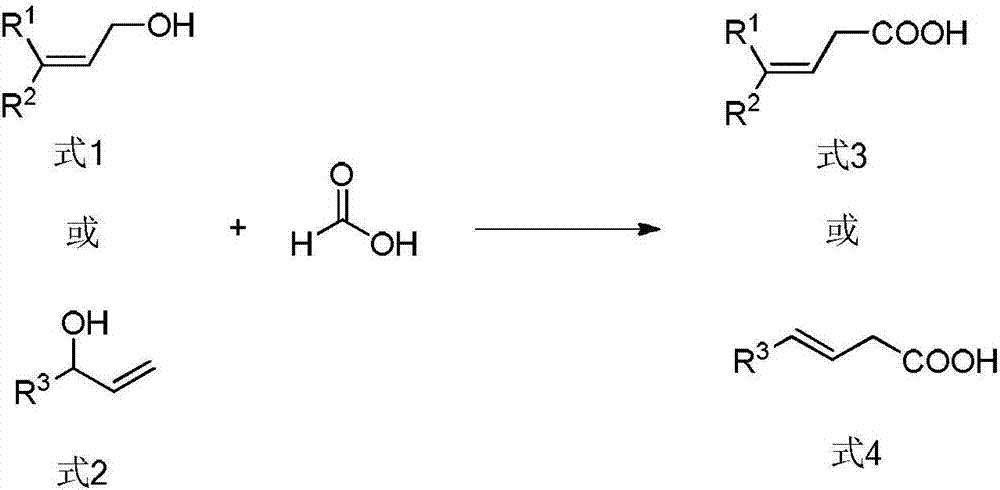
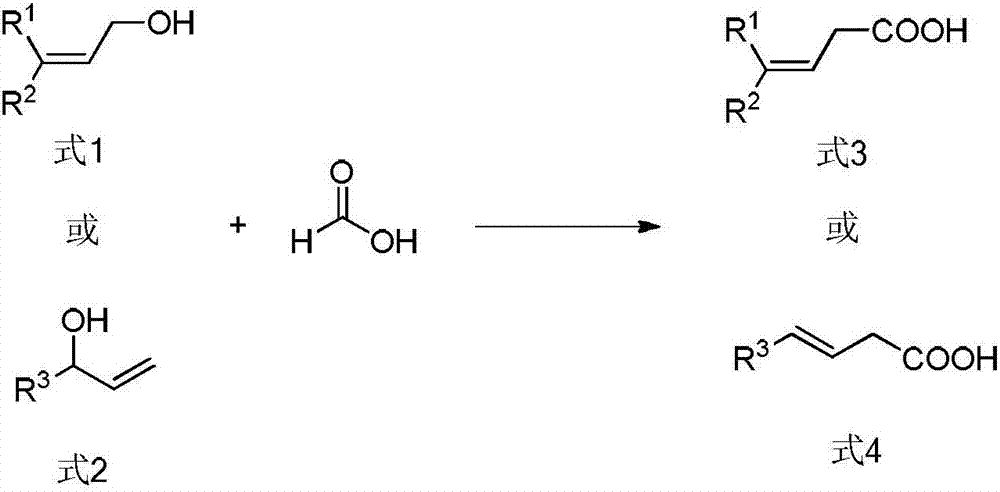
Method for preparing beta, gama-unsaturated carboxylic acid compound
A technology for compounds and carboxylic acids, applied in the field of compound synthesis, to achieve the effects of high yield, high conversion rate of reactants, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation (E)-4-phenylbut-3-enoic acid
[0042] The reaction conditions are as follows:
[0043]
[0044] The operation process is as follows:
[0045] Add tris(dibenzylidene indeneacetone)dipalladium (Pd 2 (dba) 3 , 0.5mol%, 2.3mg), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (xantphos, 2.0mol%, 5.8mg). The air in the tube was completely replaced with argon three times, and then 1 mL of toluene, cinnamyl alcohol (0.50 mmol, 67 mg), formic acid (1.5 mmol, 69 mg) and acetic anhydride (1.5 mmol, 152 mg) were added under an argon atmosphere. After the reaction tube was sealed, the reaction system was heated to 80°C in an oil bath and stirred continuously for 12 hours (IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm). After the reaction was completed, the system was cooled to room temperature. The reaction solution was diluted with ethyl acetate, and then the diluted reaction solution was concentrated by rotary evaporation (BUCHI Co., ...
Embodiment 2
[0049] Example 2: Preparation of (E)-4-(4-methoxyphenyl)but-3-enoic acid
[0050] The reaction conditions are as follows:
[0051]
[0052] The operation process is the same as that of Example 1, white solid with a yield of 78% and a purity of >99%.
[0053] The (E)-4-(4-methoxyphenyl)but-3-enoic acid obtained in Example 2 is analyzed by nuclear magnetic resonance, and the results are as follows:
[0054] 1H NMR (400MHz, CDCl3) δ7.31(d, J=8.8Hz, 2H), 6.85(d, J=8.8Hz, 2H), 6.46(d, J=15.9Hz, 1H), 6.14(dt, J =15.8,7.1Hz,1H),3.80(s,3H),3.27(dd,J=7.2,1.4Hz,2H);
[0055] 13C NMR (101 MHz, CDCl3) δ 177.8, 159.4, 133.5, 129.6, 127.6, 118.7, 114.1, 55.4, 38.1.
Embodiment 3
[0056] Example 3: Preparation of (E)-4-(4-fluorophenyl)but-3-enoic acid
[0057] The reaction conditions are as follows:
[0058]
[0059] The operation process is the same as that of Example 1, white solid with a yield of 85% and a purity of >99%.
[0060] The (E)-4-(4-fluorophenyl)but-3-enoic acid obtained in Example 3 is analyzed by nuclear magnetic resonance, and the results are as follows:
[0061] 1H NMR (400MHz, CDCl3) δ7.34 (m, 2H), 7.00 (m, 2H), 6.48 (d, J = 15.9Hz, 1H), 6.20 (dt, J = 15.8, 7.1Hz, 1H), 3.29 (dd,J=7.1,1.2Hz,2H);
[0062] 13C NMR (101 MHz, CDCl3) δ 178.2, 162.5, 133.0, 132.9, 128.0, 120.6, 115.62, 38.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com