Preparation method for health product pterostilbene
A technology for red sandalwood and health care products, applied in the field of health care products, can solve the problems of not conforming to green environmental protection, difficult to control reaction, prone to side reactions, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
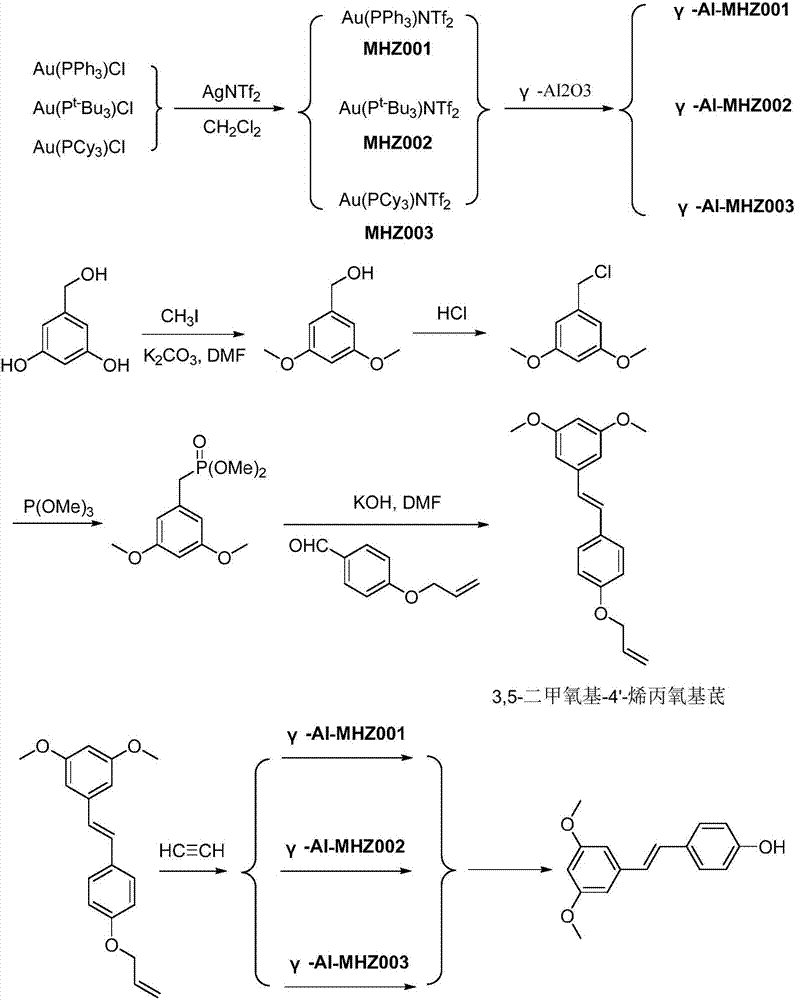
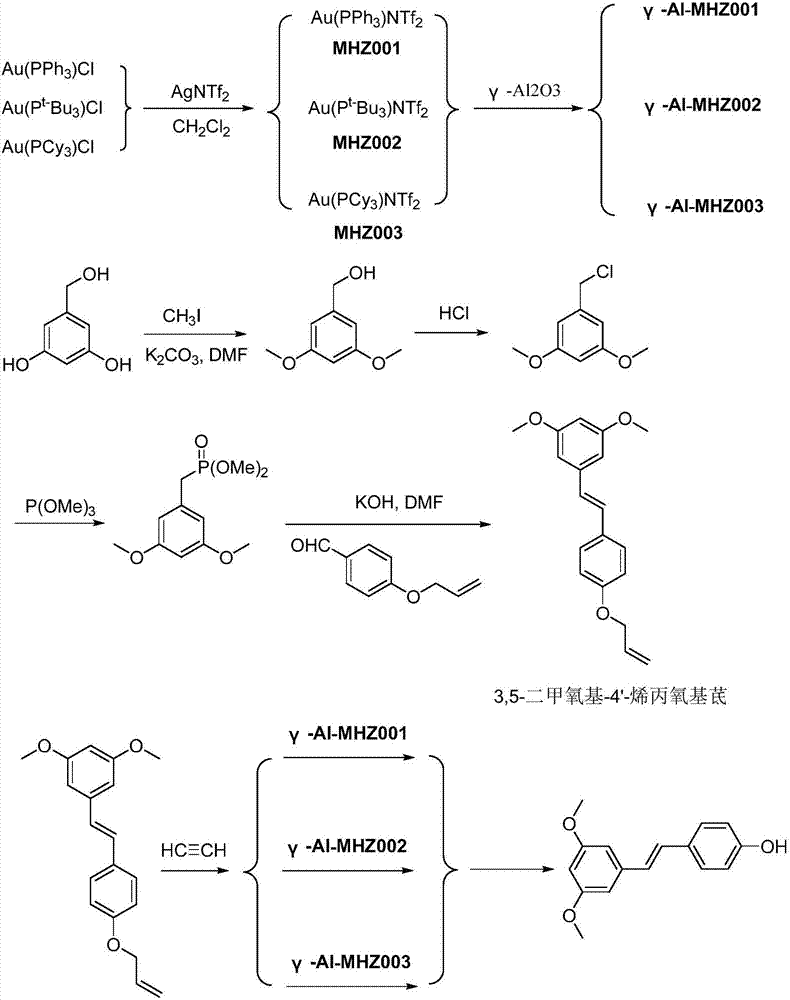
[0024] Get 4.94g triphenylphosphine gold chloride and 3.87g and two (trifluoromethanesulfonate group) silver ammonium in the reaction flask of 50ml band magnetic stirrer, after replacing reaction flask with argon gas 5 times, add 8.4g di Chloromethane, stirred at room temperature for 1 h. After completion of the reaction, suction filter the mother liquor into a new 100ml reaction flask, wash the filter cake with 8.4g of dichloromethane, and dry the by-product silver chloride; combine the filtrates to obtain a dichloromethane solution of MHZ001. Add 20g of γ-alumina to the dichloromethane solution of the obtained MHZ001, adjust the data of the rotary evaporator to 40°C, 0.01MPa, and evaporate the impregnation solution to dryness to obtain the γ-Al-MHZ001 catalyst, which can be used by recovering the dichloromethane.
[0025] Take the prepared 3,5-dimethoxy-4'-allyloxystilbene 29.6g and place it in a 250ml reaction flask with a magnetic stirrer, add 84g of dichloromethane and st...
Embodiment 2
[0027] Get 4.94g triphenylphosphine gold chloride and 3.87g and two (trifluoromethanesulfonic acid group) silver ammonium in the reaction bottle of 50ml band magnetic stirrer, after replacing reaction bottle with argon gas 5 times, add 42g dichloro methane, stirred at room temperature for 1 h. After completion of the reaction, suction filter the mother liquor into a new 100ml reaction flask, wash the filter cake with 8.4g of dichloromethane, and dry the by-product silver chloride; combine the filtrates to obtain a dichloromethane solution of MHZ001. Add 20g of γ-alumina to the dichloromethane solution of the obtained MHZ001, adjust the data of the rotary evaporator to 40°C, 0.01MPa, and evaporate the impregnation solution to dryness to obtain the γ-Al-MHZ001 catalyst, which can be used by recovering the dichloromethane.
[0028] Take the prepared 3,5-dimethoxy-4'-allyloxystilbene 29.6g and place it in a 250ml reaction flask with a magnetic stirrer, add 168g of dichloromethane ...
Embodiment 3
[0030] Get 4.34g tri-tert-butylphosphine gold chloride and 3.87g and two (trifluoromethanesulfonic acid group) silver ammonium in the reaction bottle of 50ml band magnetic stirrer, after replacing reaction bottle with argon gas 5 times, add 8.4g Dichloromethane was stirred at room temperature for 1 h. After the reaction is complete, suction filter the mother liquor into a new 100ml reaction flask, wash the filter cake with 8.4g of dichloromethane, and dry the by-product silver chloride; combine the filtrates to obtain a dichloromethane solution of MHZ002. Add 20g of γ-alumina to the dichloromethane solution of the obtained MHZ001, adjust the data of the rotary evaporator to 40°C, 0.01MPa, and evaporate the impregnation solution to dryness to obtain the γ-Al-MHZ002 catalyst, which can be used by recovering the dichloromethane.
[0031] Take the prepared 3,5-dimethoxy-4'-allyloxystilbene 29.6g and place it in a 250ml reaction flask with a magnetic stirrer, add 84g of dichloromet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com