A kind of detection method of stagnation-dispelling analgesic drug
A technology for analgesia and detection methods for Sanjie, which can be applied to measurement devices, instruments, scientific instruments, etc., and can solve the problems of labor-consuming reagents, complicated pretreatment process of detection methods, and small quantities.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
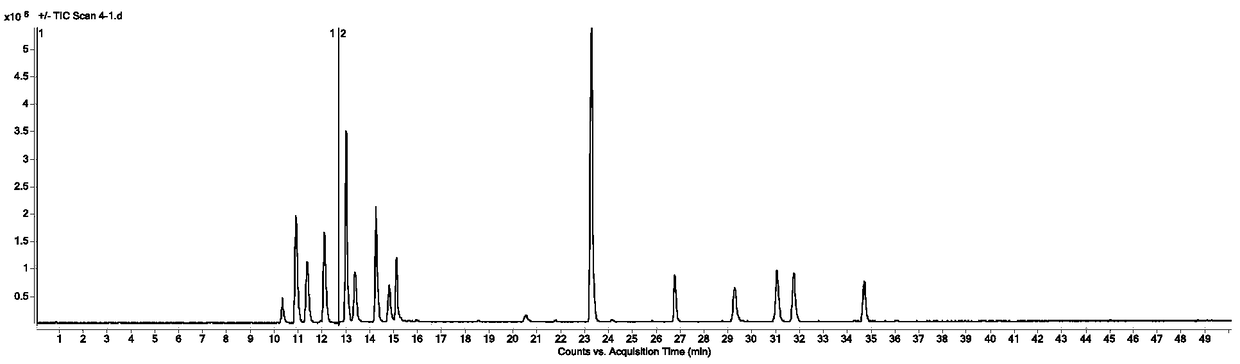
[0080] Chromatographic conditions:
[0081] Chromatographic column Agilent Zorbax SB-C18 (column length is 100mm, inner diameter is 3.0mm, particle size is 1.8μm); mobile phase is acetonitrile (A)-0.1% formic acid aqueous solution (B) gradient elution, linear elution program as shown in the table 1; flow rate: 0.4ml min-1; column temperature: 30°C; injection volume: 3μL.
[0082] Table 1: Gradient elution program of Example 1
[0083]
[0084]
[0085] Mass Spectrometry Conditions:
[0086] Using ESI source, 0~12.6min, positive ion mode, 12.6~50min, negative ion mode; capillary voltage 4000V, atomizing gas pressure 45psi, drying gas flow rate 10L / min, heating capillary temperature 350℃, source debris voltage: 210V, Skimmer 65V, mass scanning range m / z 100~2500.
[0087] Table 2 shows the extracted ion information of 14 main compound components and 3 selected internal standards in the mass-dissolving and analgesic drugs detected by the present invention for integral ca...
Embodiment 2
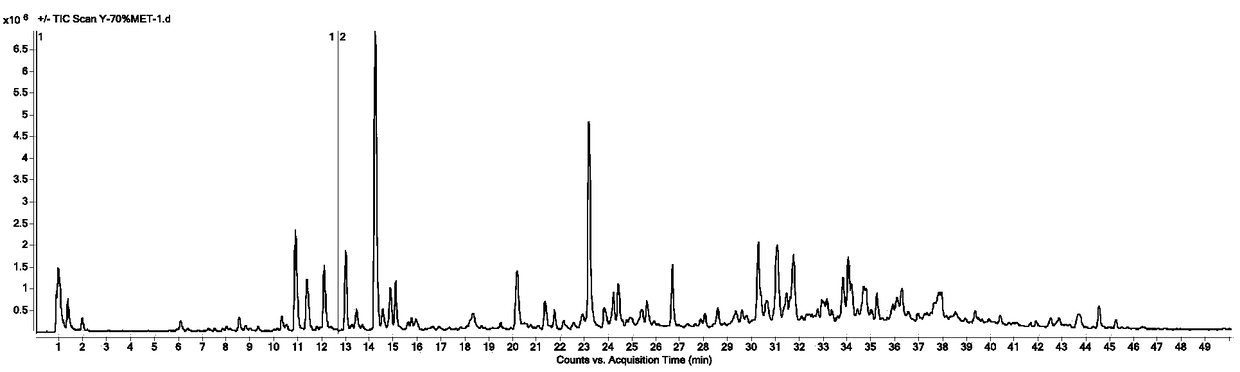
[0105] Adopt the same technical scheme of embodiment 1 to detect the fingerprint of the need testing solution that obtains, and calculate the chromatographic peak according to the fingerprint that obtains, and calculate the content of 14 kinds of main components in the need testing according to standard curve; In this embodiment, the ultrasonic extraction method is used to replace the reflux extraction method in Example 1. The ultrasonic extraction method in this embodiment is ultrasonic extraction (250W, 40KHz) for 1h.
[0106] Test results such as Figure 4 as shown, Figure 4 It is the fingerprint of the solution to be tested obtained in Example 2 of the present invention.
Embodiment 3
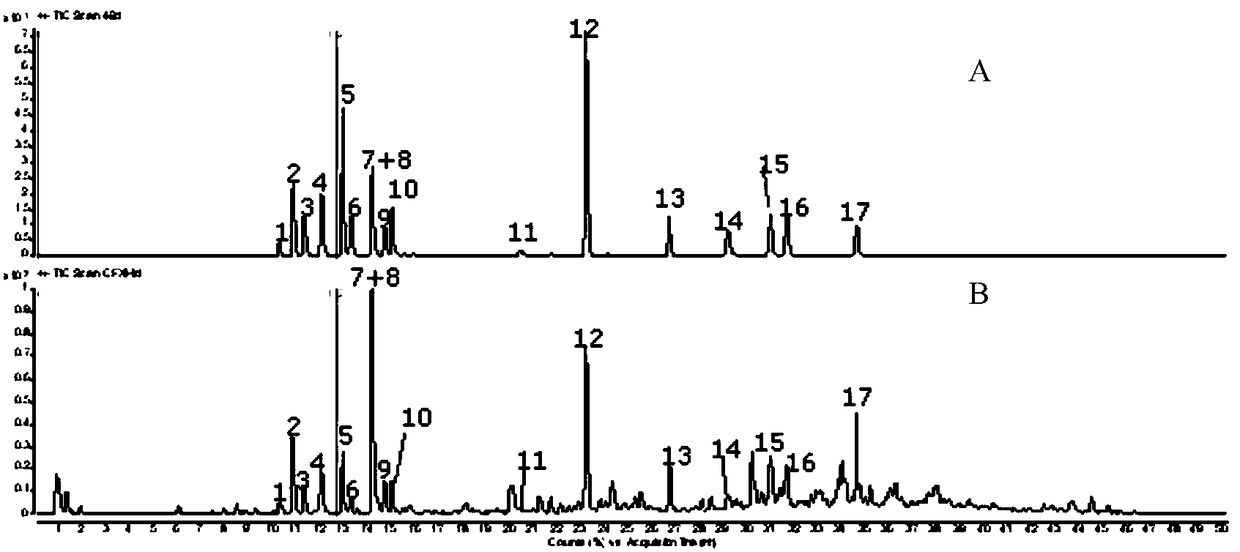
[0108] Adopt the same technical scheme of embodiment 1 to detect the fingerprint of the need testing solution that obtains, and calculate the chromatographic peak according to the fingerprint that obtains, and calculate the content of 14 kinds of main components in the need testing according to standard curve; In this embodiment, the vortex extraction method is used to replace the reflux extraction method in Example 1. The vortex extraction method in this embodiment is to vortex on a vortex mixer for 10 minutes.
[0109] Test results such as Figure 5 and as shown in Table 4, Figure 5 It is the fingerprint of the test solution obtained in Example 3 of the present invention, and Table 4 is the content of main components in the test solution obtained in Examples 1 to 3 of the present invention.
[0110] Table 4 Examples 1-3 use three kinds of extraction methods to measure the content of each component of the test product (mg / g) respectively
[0111]
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
| volume ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com