Pidotimod effervescent tablet and preparation method thereof
A technology of pidotimod and effervescent tablets, applied in the field of pharmaceutical preparations, can solve the problems of low bioavailability, and achieve the effects of easy patient acceptance, good quality stability, and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
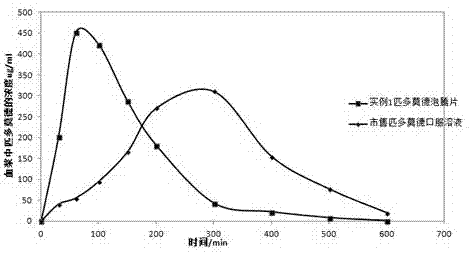
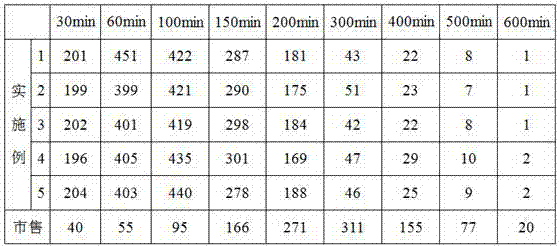
Image
Examples
Embodiment 1
[0036] Embodiment 1: 1000 pieces of specifications
[0037] prescription:
[0038] Pidotimod 380g
[0039] Microcrystalline Cellulose 150g
[0040] Lactose 50g
[0041] Citric acid 20g
[0042] Sodium bicarbonate 130g
[0044] Stevia 8g
[0045] food flavor 6g
[0047] 8% PVPK30 aqueous solution appropriate amount
[0048] Preparation method:
[0049] (1) Weigh the prescribed amount of pidotimod, microcrystalline cellulose, and lactose through an 80-mesh sieve and mix evenly;
[0050] (2) Add sodium chloride to 8% PVPK30 aqueous solution, and then add to the above powder to make soft material;
[0051] (3) Pass the soft material through a 24-mesh sieve to make granules;
[0052] (4) Put the plate into a drying oven at 60°C to dry after laying the tray, and take it out after measuring the moisture content;
[0053] (5) Add citric acid, sodium bicarbonate, stevia, food flavor, magnesium stearate and mix eve...
Embodiment 2
[0055] Embodiment 2: 1000 pieces of specifications
[0056] prescription:
[0057] Pidotimod 400g
[0058] Microcrystalline Cellulose 150g
[0059] Mannitol 50g
[0060] Citric acid 22g
[0061] Sodium bicarbonate 143g
[0062] Sodium chloride 15g
[0063] Aspartame 8g
[0064] food flavor 6g
[0066] 8% PVPK30 aqueous solution appropriate amount
[0067] Preparation method:
[0068] (1) Weigh the prescribed amount of pidotimod, microcrystalline cellulose, and mannitol and pass through an 80-mesh sieve and mix evenly;
[0069] (2) Add sodium chloride to 8% PVPK30 aqueous solution, and then add to the above powder to make soft material;
[0070] (3) Pass the soft material through a 24-mesh sieve to make granules;
[0071] (4) Put the plate into a drying oven at 60°C to dry after laying the tray, and take it out after measuring the moisture content;
[0072] (5) Add citric acid, sodium bicarbonate, aspartame, food flavor, magnesium ste...
Embodiment 3
[0074] Embodiment 3: 1000 pieces of specifications
[0075] prescription:
[0076] Pidotimod 420g
[0077] Mannitol 150 g
[0078] Lactose 50g
[0079] Citric acid 21g
[0080] Sodium bicarbonate 136.5g
[0081] Sodium chloride 15g
[0082] Stevia 8g
[0083] food flavor 6g
[0084] Talc powder 8 g
[0085] 8% PVPK30 aqueous solution appropriate amount
[0086] Preparation method:
[0087] (1) Weigh the prescribed amount of pidotimod, mannitol, and lactose through an 80-mesh sieve and mix evenly;
[0088] (2) Add sodium chloride to 8% PVPK30 aqueous solution, and then add to the above powder to make soft material;
[0089] (3) Pass the soft material through a 24-mesh sieve to make granules;
[0090] (4) Put the plate into a drying oven at 60°C to dry after laying the tray, and take it out after measuring the moisture content;
[0091] (5) Add citric acid, sodium bicarbonate, stevia, food flavor, talcum powder and mix evenly, measure the content of intermediates;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com