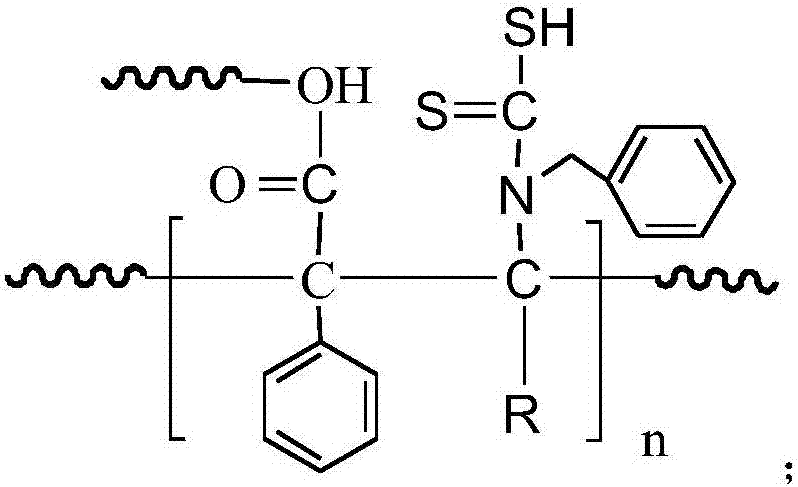
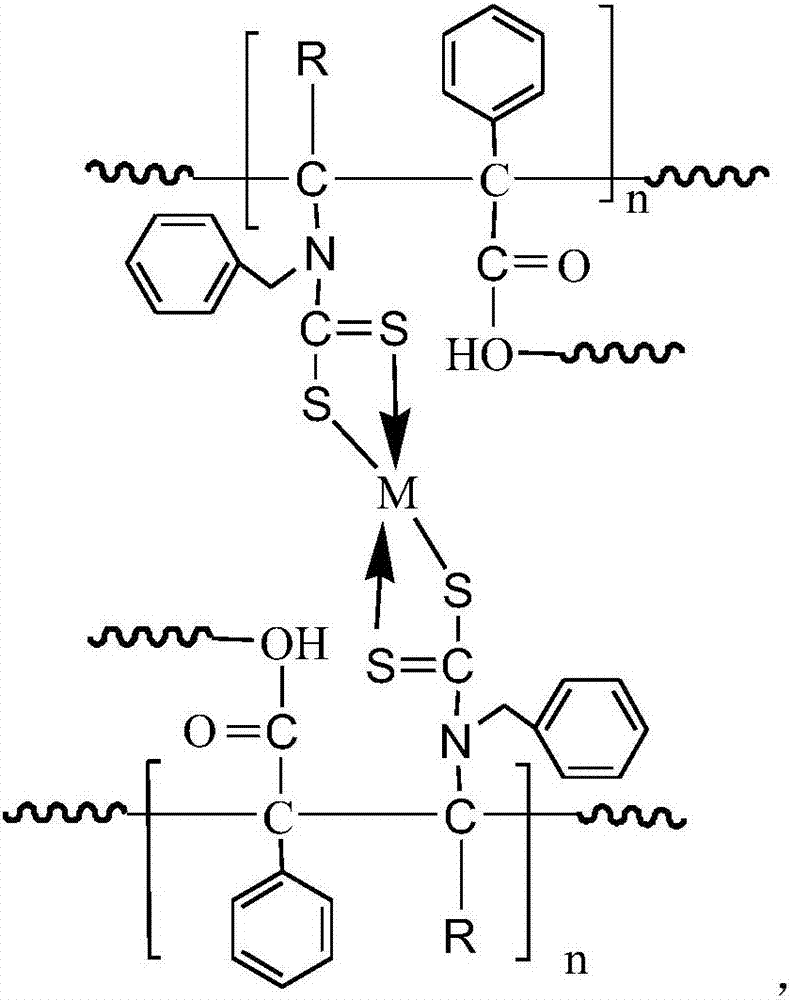
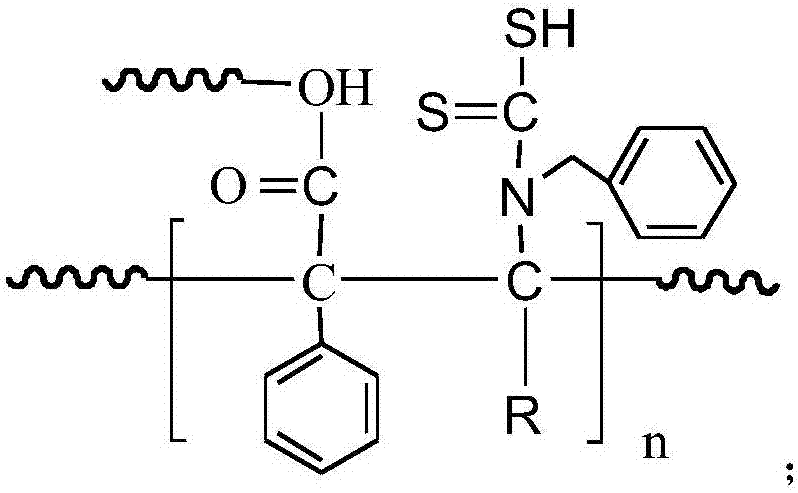
Heavy metal adsorbent and preparation method thereof
A technology of heavy metals and adsorbents, applied in the directions of alkali metal compounds, chemical instruments and methods, adsorbed water/sewage treatment, etc., can solve the problems of difficult to remove complexes, etc., and achieve the effect of simple synthesis method and strong adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 500ml of deionized water, 3.0g of gelatin, and 2.5g of methylcellulose into a 2000ml flask equipped with a stirring and temperature control device, and stir until dissolved to obtain an aqueous phase. Prepare 500 g of oil phase, including 40 g of styrene, 20 g of allyl itaconate, 40 g of allyl tripolyisocyanate, 100 g of divinylbenzene, 200 g of heptanol, and 100 g of ethyl acetate. In addition, 0.5 g of benzoyl peroxide and 1 g of azobisisobutyronitrile were added to the oil phase, mixed uniformly, and then added to the above water phase. Adjust the rotation speed until the oil phase is dispersed in the water phase to form a suitable particle size, then raise the temperature to 65°C, keep it warm for 12 hours, cool, wash with water, and dry to obtain polystyrene resin white balls.
[0036] Take 20ml of polystyrene resin white balls, 240ml of water, and 5ml of benzylamine, add them into a 500ml flask, stir at 30°C for 18h, wash with water and dry to obtain aminated ...
Embodiment 2
[0041] Add 500 ml of deionized water, 5.0 g of gelatin, and 5.0 g of methylcellulose into a 2000 ml flask equipped with a stirring and temperature control device, and stir until dissolved to obtain an aqueous phase. Prepare 600g of oil phase, including 70g of styrene, 40g of allyl itaconate, 40g of allyl tripolyisocyanate, 200g of divinylbenzene, 200g of heptanol, and 50g of ethyl acetate. In addition, 1 g of benzoyl peroxide and 1.5 g of azobisisobutyronitrile were added to the oil phase, mixed uniformly, and then added to the above water phase. Adjust the rotational speed until the oil phase disperses into a suitable particle size in the water phase, then raise the temperature to 75°C, keep it warm for 16 hours, cool, wash with water, and dry to obtain polystyrene resin white balls.
[0042] Add 20ml of polystyrene resin white balls, 210ml of water, and 5.25ml of benzylamine into a 500ml flask, stir at 40°C for 16h, wash with water and dry to obtain aminated resin microspher...
Embodiment 3
[0047] Add 500 ml of deionized water, 4.0 g of gelatin, and 3.0 g of methylcellulose into a 2000 ml flask equipped with a stirring and temperature control device, and stir until dissolved to obtain an aqueous phase. Prepare 500 g of oil phase, including 50 g of styrene, 40 g of allyl itaconate, 10 g of allyl tripolyisocyanate, 100 g of divinylbenzene, 100 g of heptanol, and 200 g of ethyl acetate. In addition, 1.5 g of benzoyl peroxide and 1 g of azobisisobutyronitrile were added to the oil phase, mixed uniformly, and then added to the above-mentioned flask. Adjust the rotation speed until the oil phase is dispersed in the water phase to form a suitable particle size, then raise the temperature to 80°C, keep it warm for 18 hours, cool, wash with water, and dry to obtain polystyrene resin white balls.
[0048]Add 10ml of polystyrene resin white balls, 150ml of water, and 3ml of benzylamine into a 500ml flask, stir at 30°C for 24h, wash with water and dry to obtain aminated resi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com