Blaps rynchopetera fairmaire extract E as well as preparation method and pharmaceutical application thereof
A technology of rostral pipa and pipa, applied in the field of pyridine compound rostral pipa and its preparation, to achieve potential social and economic benefits, convenient raw material sources, and easy-to-obtain raw material sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
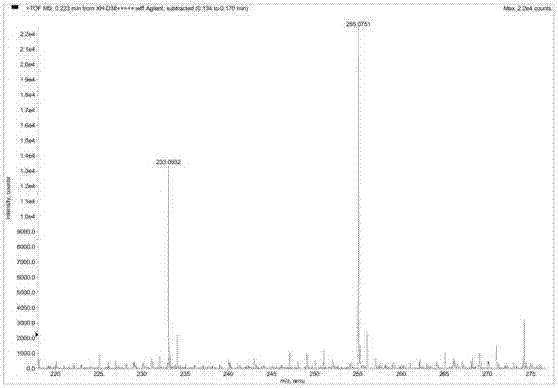
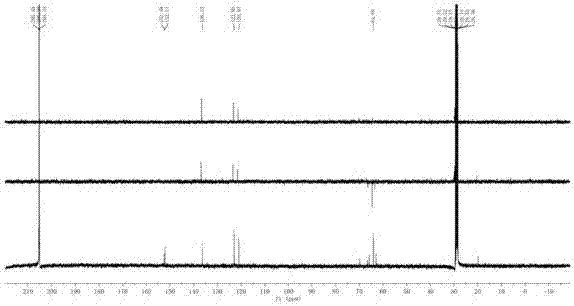
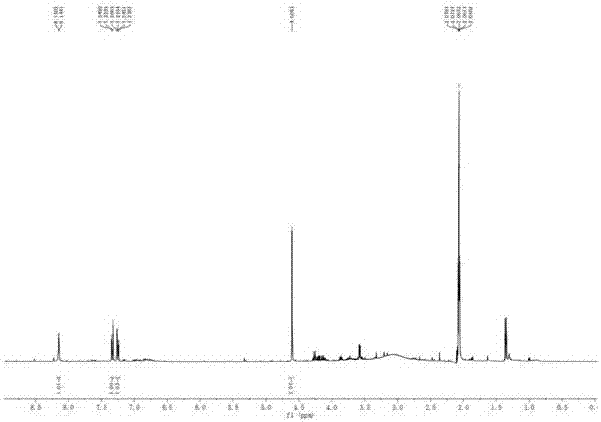
[0028] Example 1 :Preparation and structure identification of beaktail Lutein A
[0029] 1.1 Experimental equipment and materials
[0030] NMR spectrum was measured by Bruker AV-400 nuclear magnetic resonance spectrometer (δ is ppm, TMS is internal standard, J is Hz); MS spectrum is measured by Agilent G3250AA LC / MSD TOF electrospray time-of-flight mass spectrometer; Rotary evaporator uses Heidolph, Germany; the circulating condensate pump adopts Japan's EYELA; the analytical balance adopts the pioneer manufactured by Ohaus Instrument (Shanghai) Co., Ltd. Sephadex LH-20 for column chromatography was purchased from Amphamacia Biotechnology (China) Co., Ltd.; ODS reversed-phase material was purchased from Merck; column-layer silica gel and thin-layer chromatography silica gel plates were Produced by Qingdao Ocean Chemical Plant. The elution solvents for chromatography are industrially pure solvents or chemically pure solvents that have been re-evaporated; the solvent used in the d...
Embodiment 2
[0043] Example 2 :Detection of the ability of compounds of formula (I) to scavenge 1,1-diphenyl-dipicrylhydrazine (DPPH) free radicals
[0044] 2.1 Experimental principle and experimental purpose
[0045] DPPH is a relatively stable lipid free radical with a free electron on its N, and its ethanol solution is purple. Scanning at the full wavelength shows that it has a maximum absorption peak at 517nm. After adding antioxidants, DPPH captures an electron to pair with free electrons, the purple fades and becomes a colorless substance, and the absorption peak at 517nm disappears. The degree of fading has a quantitative relationship with the number of electrons it accepts. That is, the absorption value of DPPH decreases when it is redox, and the lower the absorbance, the stronger its antioxidant effect. According to this principle, the ability of the sample to provide hydrogen atoms, scavenge free radicals, and resist oxidation can be measured.
[0046] 2.2 Methods and results
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com