Application of triterpenoid saponins sourced from schima superba
A technology of triterpene saponins and compounds, applied in the direction of medical preparations containing active ingredients, cosmetics, cosmetic preparations, etc., can solve the problem of no antibacterial biological activity of Schima superba extract, and achieve good antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dry coarse powder 4.5kg of Schima superba stem, heat and reflux 3 times with 95% ethanol (8 times the amount of medicinal material), each time for 2 hours, combine the extracts for 3 times, and concentrate under reduced pressure to obtain 200g of total extract.
[0022] The total extract was dissolved and dispersed with 50% methanol water, extracted with petroleum ether, chloroform, ethyl acetate and n-butanol in sequence to obtain 50 g of petroleum ether layer, 35 g of chloroform layer, 22 g of ethyl acetate layer and 20 g of n-butanol And the water layer 45g. The ethyl acetate fraction was subjected to silica gel column chromatography in a chloroform-methanol system (100:0; 90:10; 80:20; 70:30; 60:40; 50:50; 30:70; 0:100) to obtain Fr.1 -20 fractions. Wherein fraction Fr.12 (3.3g) is subjected to ODS reverse column chromatography (180g, 3.5cm x 40cm) water-methanol system (80:20; 70:30; 60:40; 40:60; 20:80; 0 :100) to obtain fraction Fr.12-1~13. After preparative o...
Embodiment 2
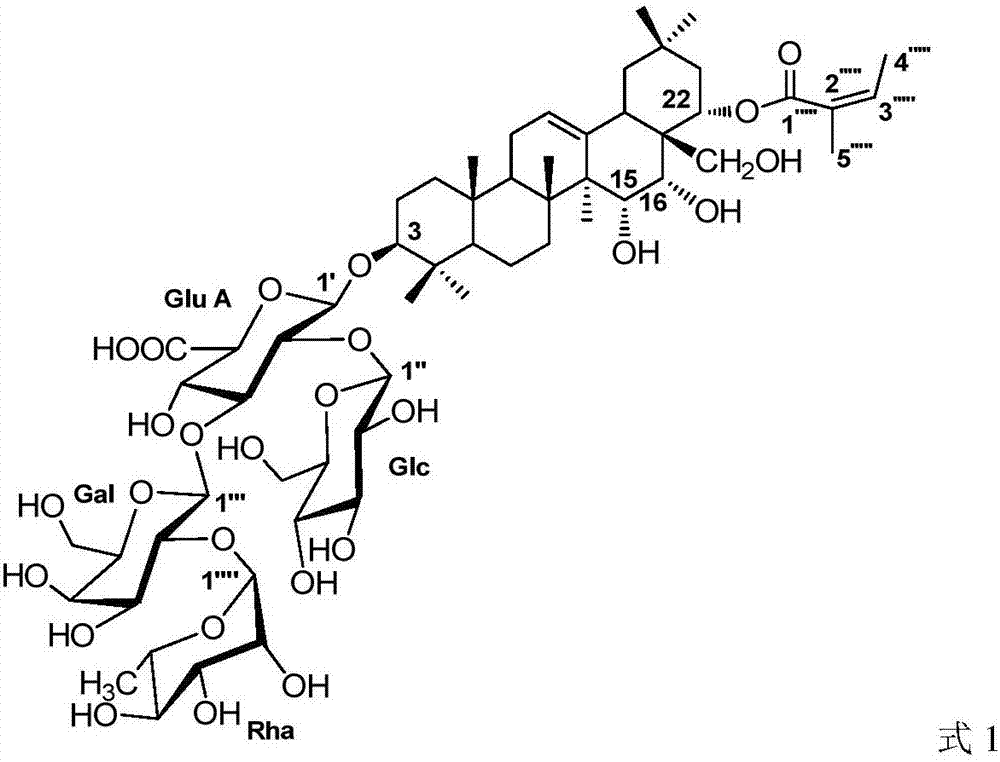
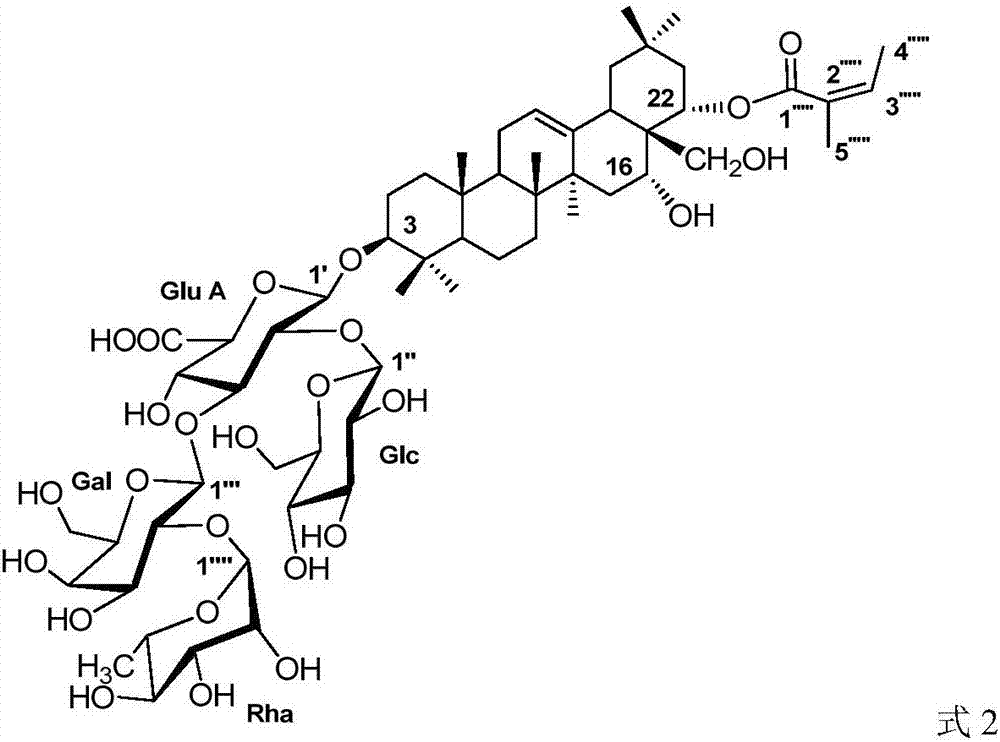
[0027] 1. Structural identification of the compound sasanquasaponin III (SS-9)
[0028]
[0029] White amorphous powder, (c 0.30, MeOH). Both Liebermann-Burchard and Molish reactions were positive, and the color of concentrated sulfuric acid-vanillin was red (TLC), which was speculated to be triterpenoid saponins. ESI-MS m / z1241.6[M+Na] + , suggesting that the molecular weight may be 1218, combined with 1 H NMR and 13 C NMR spectrum speculates that the molecular formula of compound SS-9 is C 59 h 94 o 26 .
[0030] 1 H NMR spectrum shows 7 angular methyl hydrogen signals [δ H 0.81(3H,s,H-25),1.03(3H,s,H-26),1.08(3H,s,H-29),1.08(3H,s,H-24),1.30(3H,s, H-23), 1.43(3H,s,H-30), 1.85(3H,s,H-27)], 1 ene hydrogen signal (δ H 5.48,br.s), four terminal hydrogen signals [δ H 4.92 (d, J=7.1Hz, H-1′), 5.95 (d, J=7.6Hz, H-1″), 6.19 (d, J=7.0Hz, H-1″′) and 6.26 (br. s,H-1″’)], a set of characteristic angelica acyl hydrogen signals [δ H 1.88(s, H 3 -5″″′), 2.05(d, J=6.6H...
Embodiment 3
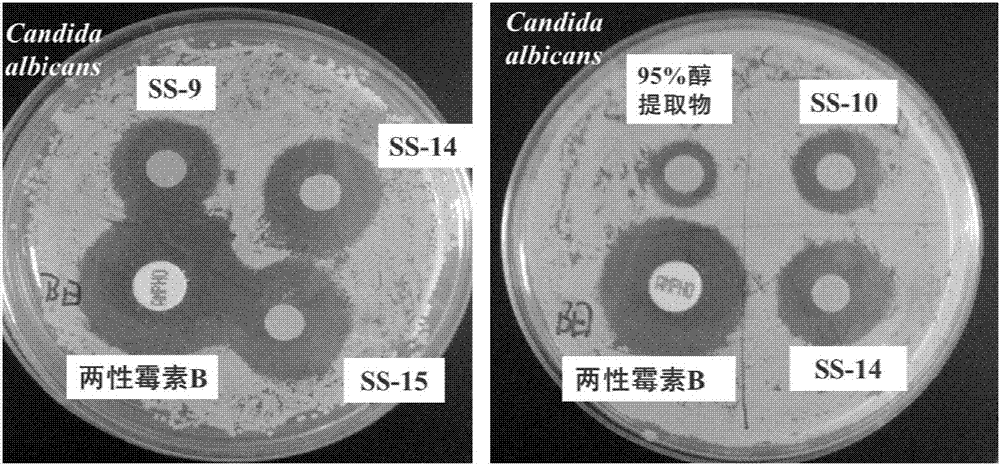
[0056] Antifungal Activity Test of Monomer Compounds in Vitro
[0057] In this experiment, sensitive strains of Candida albicans and resistant strains of Candida albicans were used as indicator bacteria, and the in vitro drug susceptibility test of the disk method was used to determine the active monomer compounds, and then the MIC value and MFC of the active monomer compounds were determined by direct visual turbidimetry. value.
[0058] 1. Instruments and experimental materials
[0059] 1.1 Main instruments and equipment
[0060] AIRTECH ultra-clean workbench (Suzhou Su Cleaning Equipment Co., Ltd.); BS200 electronic analytical balance (Sartorius, Germany); LDZX-40SC vertical self-control electric pressure steam sterilizer (Shanghai Shen'an Medical Instrument Factory); induction cooker (Zhongshan Nuojieshi Electric Co., Ltd.); 96-well cell culture plate (Coning Costar, USA); precision pipette (Gilson, France); BDS200 inverted biological microscope (Chongqing Aote Optical I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com