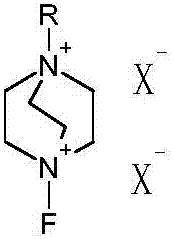Synthesis method of fluocinolone acetonide intermediate
A fluocinonide acetate and synthesis method technology, applied in the field of fluocinonide acetate intermediates, new intermediates, and fluocinonide acetate intermediates, can solve the problems of no starting material selection and process route improvement, and reduce environmental risks , the reduction of production equipment, the effect of shortening the production process and cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
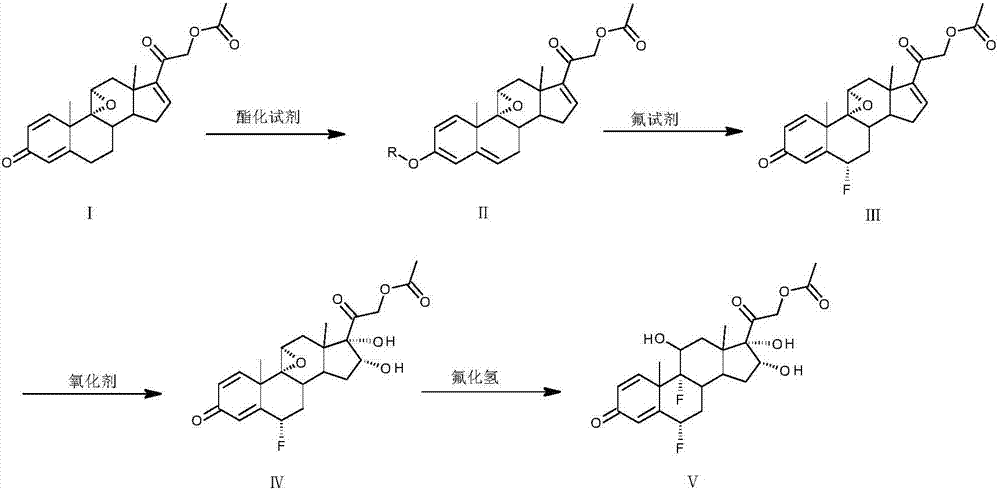
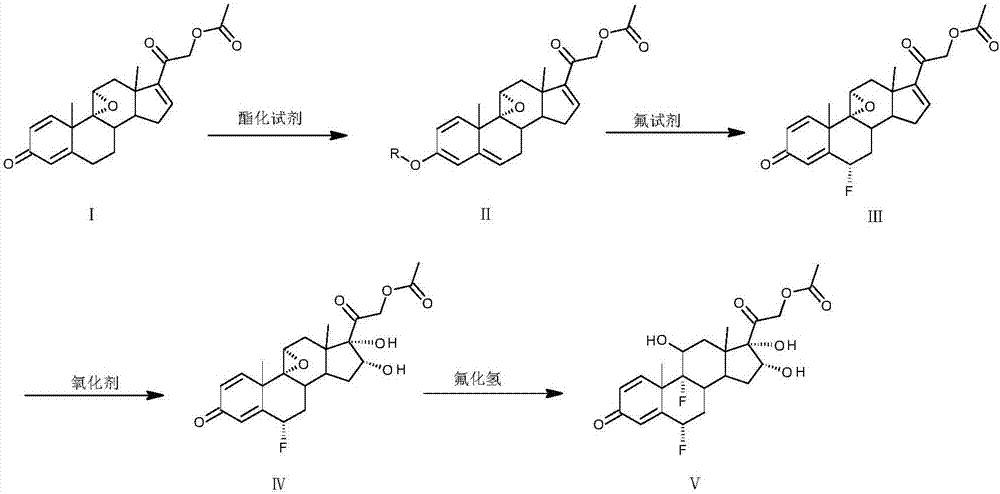
[0021] Dissolve 30 g of compound I in 100 mL of ethyl acetate, add benzoyl chloride and 1 mL of trifluoromethanesulfonic acid at 30°C, react for 1 to 5 hours, then add 5 mL of pyridine to quench, and evaporate the solvent to dryness to obtain compound II.
[0022] Dissolve compound II in 200mL dimethylformamide, add fluorine reagent at a temperature of 10°C, and react for 3 to 7 hours; wash the reaction solution into 1000mL water, extract with 100mL ethyl acetate; evaporate the ethyl acetate to dryness , to obtain compound III.
[0023] Dissolve compound III in 1000 mL of acetone, add a solution made of 20 g of potassium permanganate, 20 mL of 4M hydrochloric acid, and 400 mL of water at a temperature of 0°C, react for 1 to 3 hours, and quench with 20 g of sodium sulfite; filter, The acetone in the filtrate was evaporated to dryness, washed with water and filtered to obtain compound IV.
[0024] Put compound IV into 150mL hydrogen fluoride aqueous solution at 20°C and react f...
Embodiment 2
[0027] Dissolve 20 g of compound I in 60 mL of tetrahydrofuran, add isopropenyl acetate and 1 g of p-toluenesulfonic acid at 30°C, react for 1 to 5 hours, then add 5 mL of triethylamine to quench, and evaporate the solvent to dryness to obtain compound II.
[0028] Dissolve compound II in 140 mL of acetone, add fluorine reagent at a temperature of 10°C, and react for 3 to 7 hours; wash the reaction liquid into 1000 mL of water, extract with 50 mL of chloroform; evaporate the chloroform to dryness to obtain compound III.
[0029] Dissolve compound III in 600 mL of acetone, add a solution made of 14 g of potassium permanganate, 14 mL of 4M hydrochloric acid, and 300 mL of water at a temperature of 0°C, react for 1 to 3 hours, and quench with 14 g of sodium sulfite; filter, The acetone in the filtrate was evaporated to dryness, washed with water and filtered to obtain compound IV.
[0030] Put compound IV into 100 mL of 20°C hydrogen fluoride in dimethylformamide solution and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com