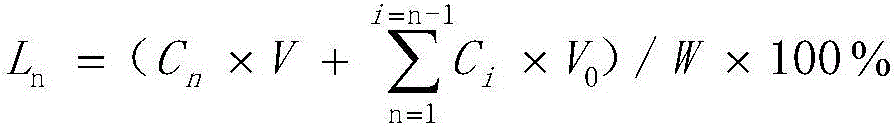External use emplastrum for treating rheumatism and relieving pain
A technology for relieving dampness and relieving pain and applying plasters, applied in the field of external plasters, which can solve the problems of difficult volatilization of active ingredients, unsatisfactory drug efficacy, and poor pertinence, and achieve the effects of relieving rheumatism pain, good transdermal absorption rate, and easy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
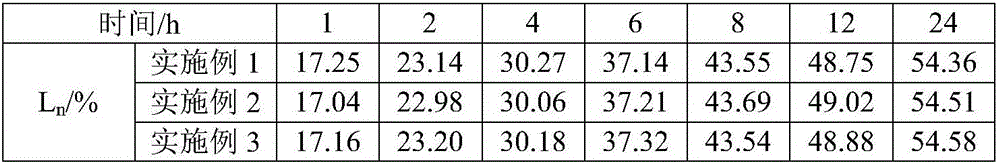
Embodiment 1
[0033] An external plaster for relieving dampness and relieving pain, prepared from the following components: musk 5g, camphor 2000g, sustained release agent 5000g, capsicum liquid extract 1500g, belladonna liquid extract 600g, methyl salicylate 800g 130g of diphenhydramine hydrochloride, 7600g of zinc oxide, 2400g of petrolatum, 6400g of rosin, 800g of lanolin, 200g of transdermal accelerator, 160g of tackifier, 100g of emulsifier, and 6800g of rubber.
[0034] Wherein the sustained-release agent is preferably nanoscale coconut shell activated carbon, the transdermal accelerator includes a main agent and an auxiliary agent, the mass percentage of the main agent is 55%, and the main agent is N,N-dimethylaminoisopropionic acid lauryl ester and The combination of ferulic acid, the mass ratio of N,N-dimethylaminoisopropionic acid dodecyl ester to ferulic acid is 1:0.3, the auxiliary agent is cocamidopropyl betaine, and the viscosity enhancer is preferably nano di For silica airge...
Embodiment 2
[0045] An external plaster for relieving dampness and relieving pain, prepared from the following components: musk 12.5g, camphor 2500g, sustained release agent 625g, capsicum liquid extract 1875g, belladonna liquid extract 750g, methyl salicylate 1000g, diphenhydramine hydrochloride 157.5g, zinc oxide 9000g, petrolatum 3000g, rosin 8000g, lanolin 1000g, skin penetration enhancer 250g, tackifier 200g, emulsifier 150g, rubber 8500g.
[0046] Wherein the sustained-release agent is preferably nanoscale coconut shell activated carbon, the transdermal accelerator includes a main agent and an auxiliary agent, the mass percentage of the main agent is 70%, and the main agent is N,N-dimethylaminoisopropionic acid lauryl ester and Combination of α-bisabolyl alcohol, the mass ratio of N,N-dimethylaminoisopropionate dodecyl to α-bisabolyl alcohol is 1:0.7, and the auxiliary agent is trioctylmethyl chloride ammonium, the tackifier is preferably nano-silica aerogel, and the emulsifier is pr...
Embodiment 3
[0057] An external plaster for relieving dampness and relieving pain, prepared from the following components: musk 20g, camphor 3000g, sustained release agent 750g, capsicum liquid extract 2250g, belladonna liquid extract 900g, methyl salicylate 1200g 185g of diphenhydramine hydrochloride, 10400g of zinc oxide, 3600g of petrolatum, 9600g of rosin, 1200g of lanolin, 300g of transdermal accelerator, 240g of tackifier, 200g of emulsifier, and 10200g of rubber.
[0058] Wherein the sustained-release agent is preferably ethyl cellulose, the transdermal enhancer includes a main agent and an auxiliary agent, the mass percentage of the main agent is 85%, and the main agent is N,N-dimethylaminoisopropionic acid dodecyl ester and glycerin Monohexyl ether, the mass ratio of dodecyl N,N-dimethylaminoisopropionate to glycerol monohexyl ether is 1:1.3, the auxiliary agent is cocamidopropyl betaine, and the tackifier is preferably nano-dioxide Silica airgel, preferably phosphatidylserine.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com