PH-sensitive mixed micelle as well as preparation method and application thereof
A mixed micellar and sensitive technology, applied in the direction of drug combination, pharmaceutical formulation, organic active ingredients, etc., can solve the problems of multidrug resistance, low bioavailability of chemotherapy drugs, etc., and achieve long drug circulation time and good size Effect of tissue retention, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
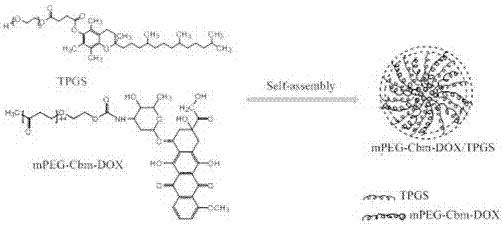
[0045] figure 1 It is a schematic diagram of the pH-sensitive polymer prodrug of the embodiment of the present invention. from figure 1 It can be seen that the pH-sensitive mixed micelles of the present invention have a double-layer structure, the outer layer is PEG with good hydrophilicity, the inner layer is hydrophobic drug DOX, and TPGS is also connected to it by amphiphilicity.
[0046] (a) Preparation of pH-sensitive polymeric prodrugs
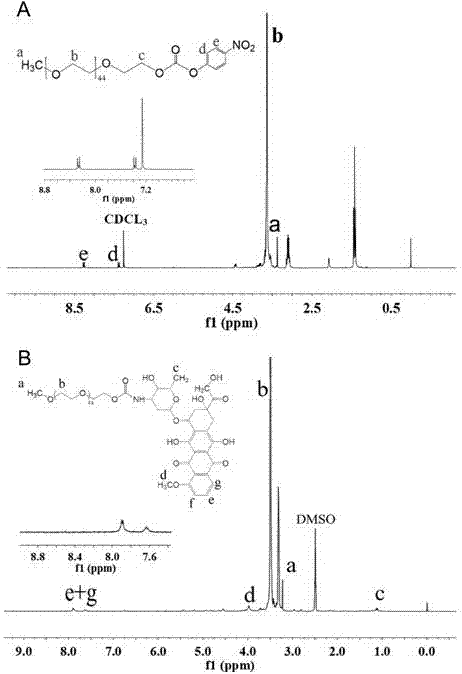
[0047] With 20 mL of anhydrous DCM (dichloromethane) as the solvent and 694 μL of TEA as the catalyst, 2.00 g of mPEG with a molecular weight of 2000 was dissolved and then added TEA dropwise, and 1.0078 g of chloroformic acid was dissolved in 10 mL of anhydrous DCM as the solvent. After the phenyl phenyl ester was dissolved, it was added dropwise at 0°C, and reacted at 30°C for 24 hours. The solution was rotary evaporated at 28°C to remove DCM, anhydrous ether was added, the precipitated white substance was collected, reprecipitated ...
Embodiment 2
[0054] The particle size distribution characterization of the polymer prodrug and a pH-sensitive mixed micelle, the specific steps are as follows:
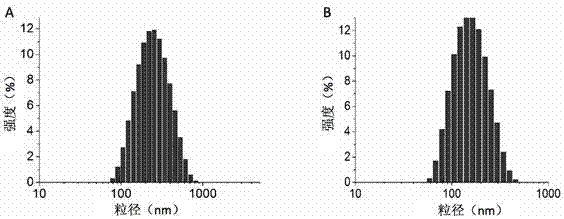
[0055] The polymer prodrug was made into a solution with a concentration of 500 μg / mL, and the polymer prodrug and TPGS were mixed at a molar ratio of 3:1. Measurement. The results of polymer prodrug testing are as follows: image 3 Shown in A, the average particle diameter of this polymer prodrug micelle is 204 nm, and PDI is 0.232; The detection result of mixed micelle is as follows image 3 As shown in B, the particle size distribution is uniform, the average particle size is 144 nm, and the PDI is 0.187. The particle size distribution of this mixed micelle is narrow, and it is significantly different from the particle size of the polymer prodrug, which indicates that a new Micellar rather than physical mixing of polymeric prodrug and TPGS.
Embodiment 3
[0057] Morphological characterization of polymer prodrugs and a pH-sensitive mixed micelle, the specific steps are as follows:
[0058] The polymer prodrug and a pH-sensitive mixed micelle were formulated into a 100 μg / mL solution with distilled water, dropped onto the copper grid, and dried overnight (14 hours). The morphology was observed with a transmission electron microscope. From the results of transmission electron microscopy, we can see that the polymer prodrug presents a spherical morphology with good monodispersity ( Figure 4 A), pH-sensitive mixed micelles exhibit a spherical structure with good monodispersity ( Figure 4 B).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com