Organic fluorescent sensing material capable of selectively detecting nerve agent, and preparation method and application thereof
A fluorescent sensing, organic technology, applied in luminescent materials, material analysis by optical means, material analysis, etc., can solve problems such as false positives, and achieve the effect of reducing detection limit, surface pores, and specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
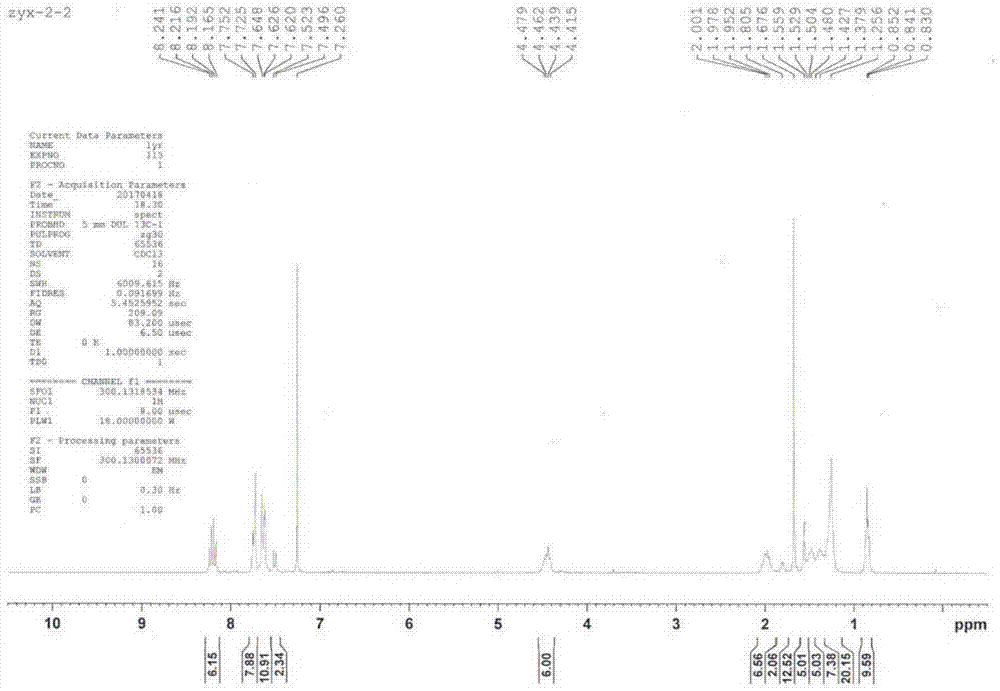
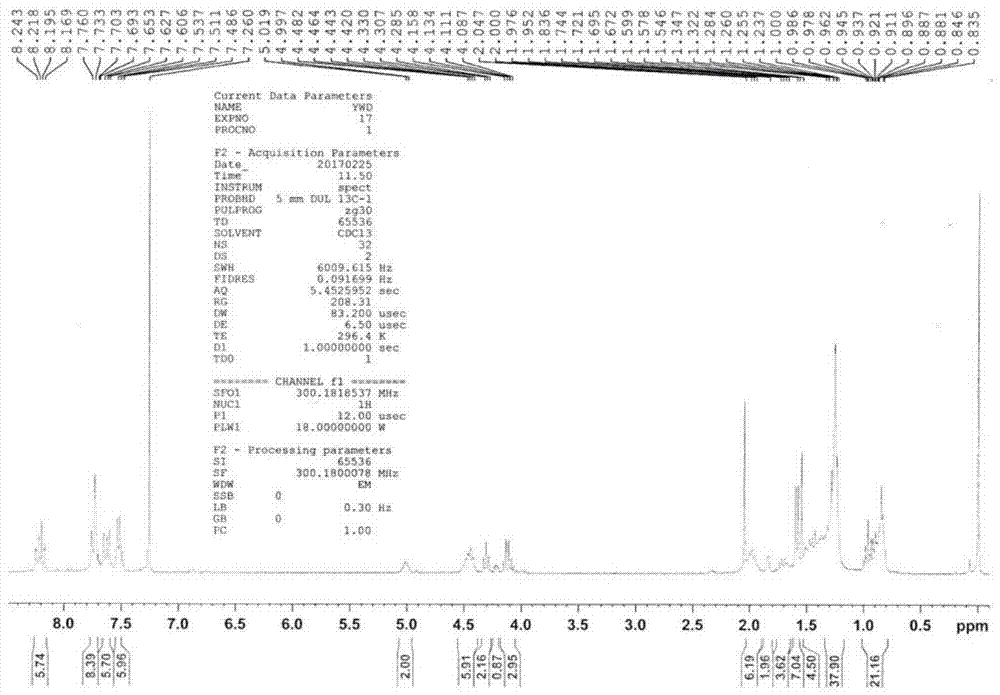
[0098] As mentioned above, the present invention discloses a method for preparing an organic fluorescent sensor material that can selectively detect nerve agents, which comprises the following steps: (1) synthesizing a carbazole derivative shown in formula (I); ( 2) self-assembling the carbazole derivative represented by formula (I) obtained in step (1) in a mixture of good solvent and poor solvent through π-π interaction to obtain the organic fluorescent sensing material.
[0099] In a preferred embodiment of the present invention, the carbazole derivative of n=3 in the preparation formula (I), said step (1) specifically comprises:
[0100] Step (1a): the compound shown in formula (II) reacts with RX' to prepare the compound shown in formula (III);
[0101]
[0102] X in formula (II) and formula (III) is the same or different, and is independently selected from halogen (such as Br, I); X' in RX' is selected from halogen (such as Br, I); Formula (III) and The definition of...
Embodiment 1
[0149] Preparation compound 1, n is the carbazole derivative of 3, and its preparation method is as follows:
[0150]
[0151] (1) Dissolve 1 g of 2,7-dibromocarbazole in 30 ml of N,N-dimethyl-formamide (DMF) solution, place the above solution in an ice bath at 0°C, and slowly add 1.2 The equivalent of 74 mg of sodium hydride solid was continuously stirred for half an hour, then 1.5 equivalent of 1-bromooctane was slowly added, and after reacting overnight at room temperature, the product was obtained by column chromatography.
[0152] (2) Get 500 mg of the product obtained in step (1), add 20 ml of 1,4-dioxane solution, add 5 equivalents of bisvaleryl diboron, 14 equivalents of potassium acetate, 10% equivalents of [1,1 '-bis(diphenylphosphino)ferrocene]palladium dichloride was reacted for 6 hours at 80°C under the protection of argon, and the product (TM-1) was obtained by column chromatography.
[0153] (3) Take 500mg of the product obtained in step (1), add 20ml 1,4-di...
Embodiment 2
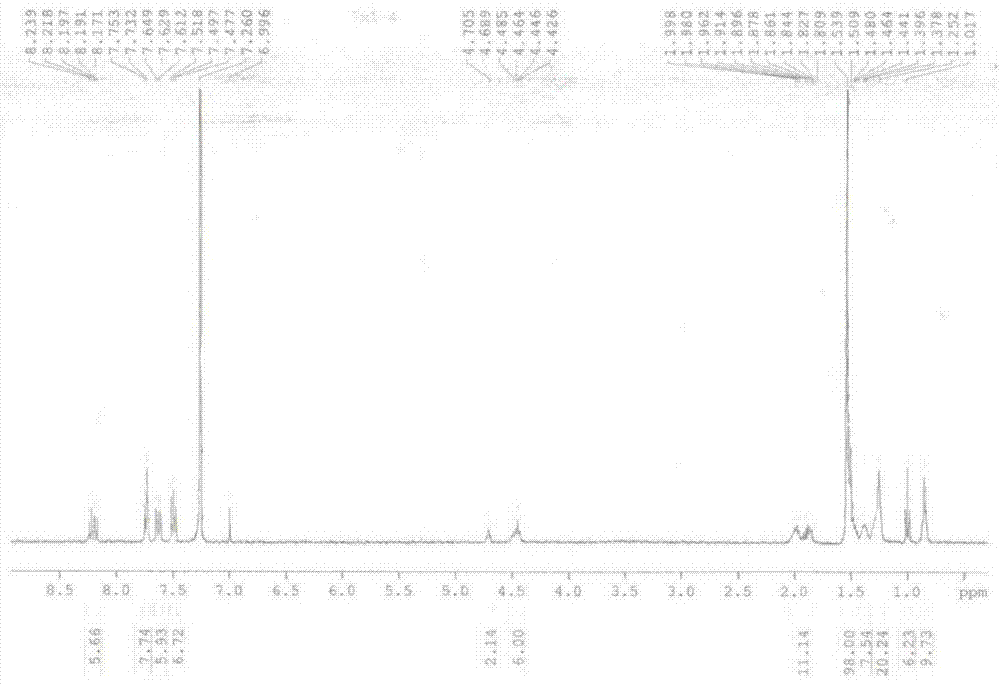
[0157] Prepare following compound 2, n is the carbazole derivative of 3, and its preparation method is as follows:
[0158]
[0159] (1) Dissolve 1 g of 2,7-dibromocarbazole in 30 ml of N,N-dimethyl-formamide (DMF) solution, place the above solution in an ice bath at 0°C, and slowly add 1.2 The equivalent of 74 mg of sodium hydride solid was continuously stirred for half an hour, then 1.5 equivalent of 1-bromooctane was slowly added, and after reacting overnight at room temperature, the product was obtained by column chromatography.
[0160] (2) Get 500 mg of the product obtained in step (1), add 20 ml of 1,4-dioxane solution, add 5 equivalents of bisvaleryl diboron, 14 equivalents of potassium acetate, 10% equivalents of [1,1 '-bis(diphenylphosphino)ferrocene]palladium dichloride was reacted for 6 hours at 80°C under the protection of argon, and the product (TM-1) was obtained by column chromatography.
[0161] (3) Dissolve 2g of 1-(4-bromophenyl)acetone in 30ml of methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com