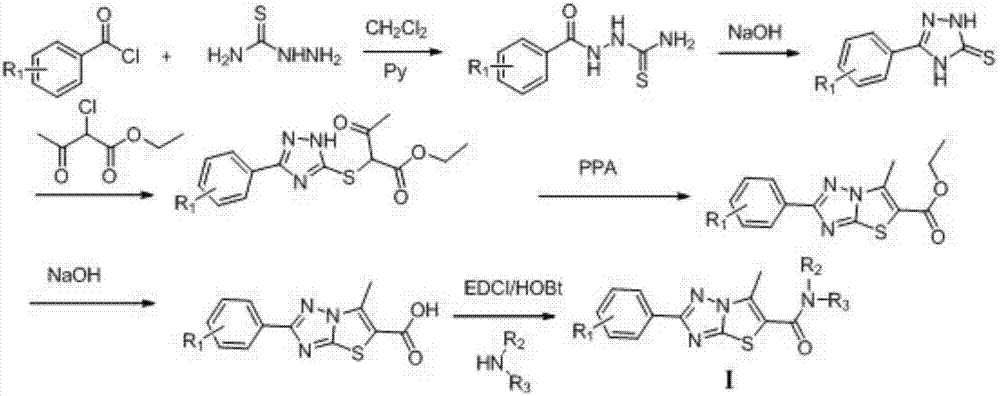
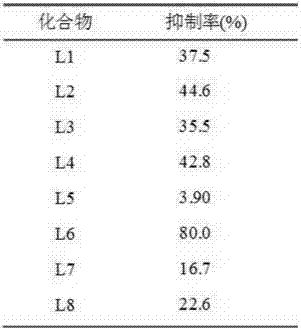
6-methyl-thiazole triazole-5-formamide derivative and application
A technology of triazole and thiazolide, applied in the field of medicine, can solve the problems of tumor tissue degeneration, inability to meet the needs of tumor survival and growth, and inability of nutrients to reach tumor cells through diffusion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of 2-(4-chlorophenyl)-6-methyl-thiazole[3,2-b][1,2,4]triazole-5-carboxylic acid
[0017] Add 0.1 mol of thiosemicarbazide and 100 mL of dichloromethane into a 250 mL three-necked flask, stir to dissolve in an ice water bath, and then add 0.13 mol of pyridine. Slowly add 0.13 mol of 4-chlorobenzoyl chloride dropwise at 0-5°C for 20 minutes to complete the dropwise addition, and react at 15°C for 2 hours to complete the reaction. A large amount of white solid appears in the system, filter. The obtained white solid was dissolved in 80 mL of 5% by mass sodium hydroxide solution, heated to reflux for 2h, and cooled to room temperature. The pH was adjusted to 5-6 with 3.65% by mass dilute hydrochloric acid. A large amount of light yellow solid precipitated out and filtered. Recrystallize to obtain 18.3 g of 5-(4-chlorophenyl)-3-mercapto-1,2,4-triazole, with a yield of 86.7%, ESI-MS (m / z): 212.3 (M+H) + .
[0018] Add 0.06mol of 5-(4-chlorophenyl)-3-mercapto-1,2,4-tri...
Embodiment 2
[0022] N,N-Dimethyl-2-(4-chlorophenyl)-6-methyl-thiazolo[3,2-b][1,2,4]triazol-6-yl}-5- Preparation of formamide (L1) 10mmol dimethylamine hydrochloride, 10mmol 2-(4-chlorophenyl)-6-methyl-thiazole [3,2-b][1,2,4]triazole-5 -Formic acid, 20mL of dichloromethane were added to the round bottom flask, and then added 12mmol EDCI, 12mmol HOBt and 10mmol triethylamine, after 12h at room temperature, the reaction solution was used 5% HCl, 5% NaHCO 3 , Washed with saturated NaCl solution, dried and separated by column chromatography to obtain a white solid with a yield of 34%; 1 H-NMR (300MHz, DMSO), δ (ppm): 7.42 (2H, d, J = 8.7 Hz), 7.26 (2H, d, J = 8.7 Hz), 3.96 (3H, s), 3.02 (3H, s) ), 2.60(3H,s); ESI-MS(m / z): 321.2(M+H) + .
Embodiment 3
[0024] N,N-Diethyl-2-(4-chlorophenyl)-6-methyl-thiazolo[3,2-b][1,2,4]triazole-5-carboxamide (L2) Preparation
[0025] The dimethylamine hydrochloride was replaced with diethylamine, the synthesis method was referred to Example 2, and the yield was 36%.
[0026] 1 H-NMR (300MHz, DMSO), δ (ppm): 7.52 (2H, d, J = 9.0 Hz), 7.37 (2H, d, J = 9.0 Hz), 3.98 (2H, m), 3.32 (2H, m ),2.30(3H,s),1.18(3H,t),1.05(3H,t); ESI-MS(m / z):349.1(M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com