Method for preparing high-purity lithium oxide from lithium carbonate
A technology of lithium oxide and lithium carbonate, applied in the direction of lithium oxide;/hydroxide, etc., can solve problems such as low production efficiency, long time, and incomplete decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
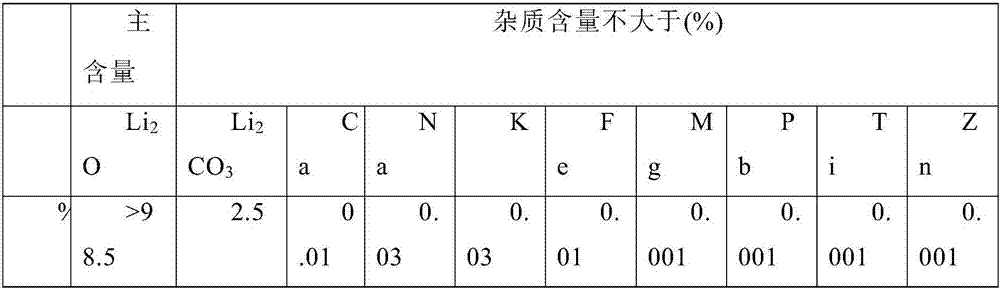
[0034] A, acidification and neutralization: with 1kg battery grade Lithium Retard (wherein each material mass percentage content is as follows Li 2 CO 3 : 99.6%, Fe: 0.0001%, Ca: 0.0019%, K: 0.0014%, Na: 0.016%, Mg: 0.0003%, Pb: <0.0001%, Ti: <0.0001%, Zn: <0.0001%)) placed In the reaction vessel, add concentrated nitric acid with a concentration of 63% while stirring, and finally control the pH of the mixed solution to 6.5 by adding lithium hydroxide and nitric acid dropwise;
[0035] B. Filtration of impurities: filter the mixed solution obtained in step A to obtain the primary filtration solution, then dropwise add lithium hydroxide and nitric acid to make the pH of the primary filtration solution reach 11.5, filter again to obtain the secondary filtration solution, and finally add Lithium hydroxide and nitric acid make the pH of the secondary filtration solution reach 1.5, and obtain the adjusted solution;
[0036] C, crystallization, separation and drying: the adjustmen...
Embodiment 2
[0039] A, acidification and neutralization: with 1.5kg battery-grade lithium carbonate (wherein each material mass percent content is as follows Li 2 CO 3 : 99.6%, Fe: 0.0001%, Ca: 0.0019%, K: 0.0014%, Na: 0.016%, Mg: 0.0003%, Pb: <0.0001%, Ti: <0.0001%, Zn: <0.0001%) placed in the reaction In the container, add concentrated nitric acid with a concentration of 63% while stirring, and finally control the pH of the mixed solution to 7 by adding lithium hydroxide and nitric acid dropwise;
[0040] B. Filtration and impurity removal: filter the mixed solution obtained in step A until the initial filtration solution, then make the pH of the primary filtration solution reach 12 by dropwise adding lithium hydroxide and nitric acid, and then filter again to obtain the secondary filtration solution, and finally pass the dropwise Add lithium hydroxide and nitric acid to make the pH of the secondary filtration solution reach 2, and obtain the adjusted solution;
[0041] C. Crystallizat...
Embodiment 3
[0044] A, acidification and neutralization: with 2kg battery grade Lithium Retard ((wherein each substance mass percent content is as follows Li 2 CO 3 : 99.6%, Fe: 0.0001%, Ca: 0.0019%, K: 0.0014%, Na: 0.016%, Mg: 0.0003%, Pb: <0.0001%, Ti: <0.0001%, Zn: <0.0001%) placed in the reaction In the container, add concentrated nitric acid with a concentration of 50-70% while stirring, and finally drop lithium hydroxide and nitric acid to control the pH of the mixed solution to 7.5.
[0045] B. Filtration and impurity removal: filter the mixed solution obtained in step A to obtain the primary filtration solution, then dropwise add lithium hydroxide and nitric acid to make the pH of the primary filtration solution reach 12.5, filter again to obtain the secondary filtration solution, and finally add Lithium hydroxide and nitric acid make the pH of the secondary filtration solution reach 2.5 to obtain the adjusted solution.
[0046] C. Crystallization, separation and drying: Evaporat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com