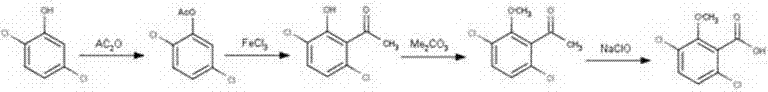
Synthesis method for raw material drugs of dicamba
A synthesis method and technology of dicamba, which is applied in the field of synthesis of dicamba technical, can solve the problems of cumbersome post-processing operations, serious environmental pollution, and high catalyst cost, and achieve easy availability of raw materials and catalysts, high reaction yield, and post-processing Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A method for synthesizing the original drug of dicamba,
[0022] Specific steps are as follows:
[0023] Add 81.5g (0.5mol) of 2,5-dichlorophenol and 200mL of tetrahydrofuran to a four-necked flask equipped with stirring, thermometer, dropping funnel, and reflux condenser (with calcium oxide drying tube), stir well, and add 61.25 g (0.6mol) acetic anhydride, slowly heated to 60-80°C, and reacted for 1.5h. After the reaction is complete, add ice water to cool down, filter, and wash with ice water to obtain a white solid that is 2,5-dichlorophenol acetate. The content is 94.4%, and the yield is 92.8%.
[0024] Add the product from the previous step (2,5-dichlorophenol acetate), 150 mL of nitrobenzene, and anhydrous ferric chloride into the three-necked flask, start stirring, slowly raise the temperature to 120°C, and react for 5 hours. After the reaction, the reaction solution was poured into a beaker filled with ice water and concentrated hydrochloric acid, and stirre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com