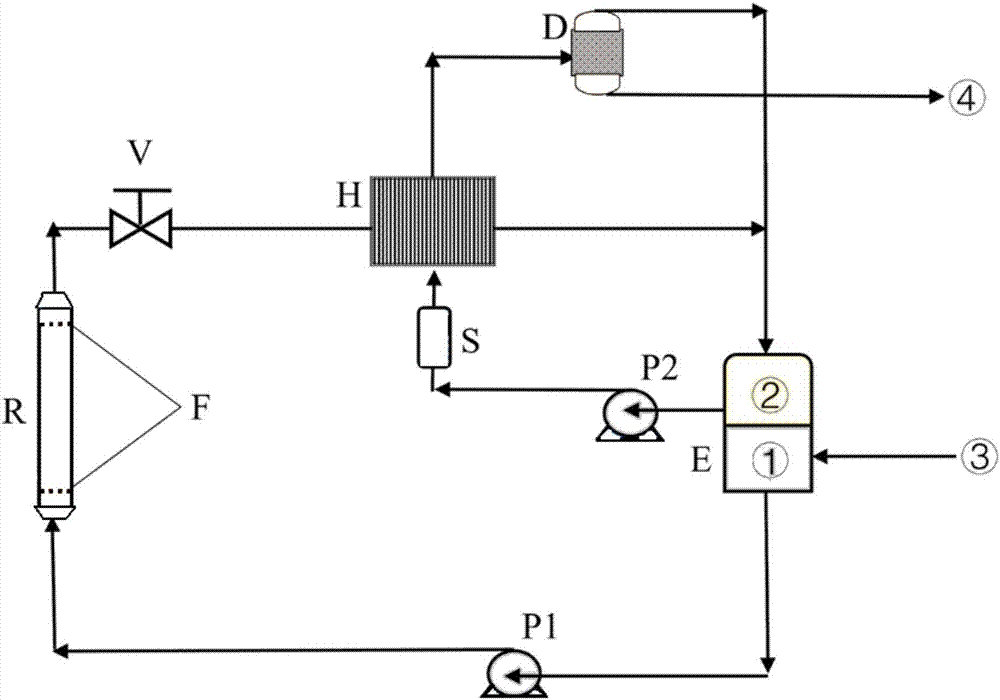
Method and system for continuously preparing 5-hydroxymethylfurfural and derivative thereof
A technology of hydroxymethyl furfural and alkoxymethyl furfural, applied in chemical instruments and methods, separation methods, chemical/physical processes and other directions, can solve the problems affecting the high yield generation of target products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] 5-HMF was prepared by using fructose as raw material and HCl as catalyst.
[0102] An acid and alkali-resistant steel pipe with outer diameter OD=15.8mm, pipe wall thickness d=1.25mm, and pipe volume V=20ml is selected as the reaction device. The polar reaction phase consisted of 28ml water + 12ml DMSO. The non-polar extract phase consisted of 24ml methyl isobutyl ketone + 16ml 2-butanol. The concentration of fructose in the polar reaction phase was 5 wt.%. The concentration of HCl in the polar reaction phase is 0.2mol / L. The flow rate of the reaction solution was 10 ml / min (empty time = 2 min). The reaction temperature is 170° C., the reaction time is 30 minutes, and the reaction column pressure is 0.1 Mpa. The polar reaction liquid undergoes a full cycle reaction in the reaction device and the extraction device. After the reaction, the conversion rate of fructose was detected by high performance liquid chromatography to be 99%, and the selectivity of 5-HMF was 93...
Embodiment 2
[0104] 5-HMF was prepared with fructose as raw material and H3PO4 as catalyst.
[0105] Choose the same reaction device and reaction conditions as in Example 1. Change the catalyst to 0.2mol / L H3PO4. After the reaction, the conversion rate of fructose was detected by high performance liquid chromatography to be 98%, and the selectivity of 5-HMF was 91%.
Embodiment 3
[0107] 5-HMF was prepared with fructose as raw material and phosphotungstic acid as catalyst.
[0108] Choose the same reaction device and reaction conditions as in Example 1. Change the catalyst to 0.2mol / L phosphotungstic acid. After the reaction, the conversion rate of fructose was detected by high performance liquid chromatography to be 100%, and the selectivity of 5-HMF was 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com