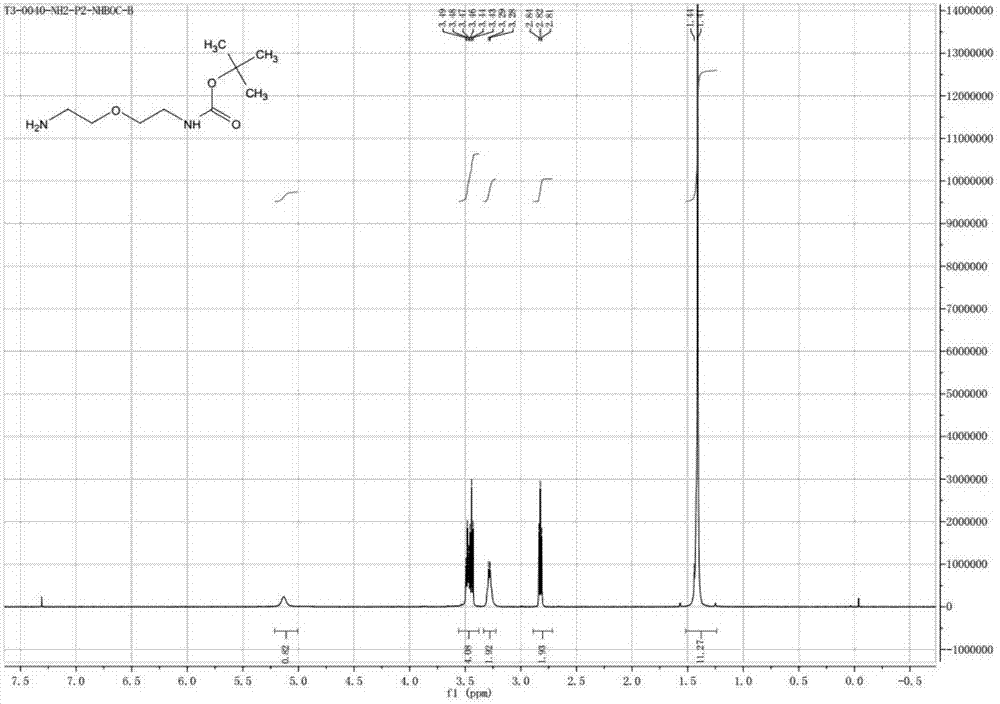
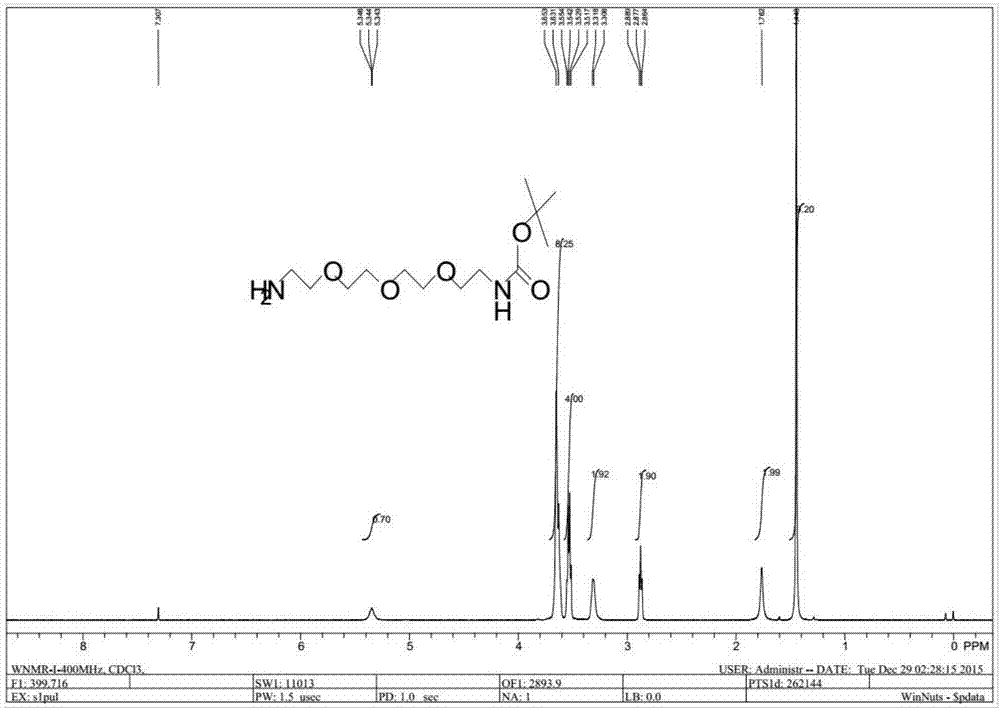
Preparation method of NH2-PEG-NHBoc
A technology of polyethylene glycol carbamic acid and polyethylene glycol, applied in the field of preparation of amino-terminated polyethylene glycol tert-butyl carbamate, can solve the problems of low raw material utilization rate, difficult post-processing, unfavorable production, etc., and achieve Conducive to extraction and purification, optimization of synthetic routes, and cost-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Add 50g of diethylene glycol and 105g of triethylamine to 500ml of dichloromethane, stir, weigh 198g of p-toluenesulfonyl chloride and dissolve in 250ml of dichloromethane, add dropwise to the reaction system under ice-water bath conditions, dropwise After completion, react for 12h at 20°C. TLC showed that the reaction was over, 400ml of water was added to wash, liquid separation, the organic phase was dried with anhydrous sodium sulfate, spin-dried, and then separated and purified by column chromatography to obtain 175g of Intermediate A, the yield: 89%. The nuclear magnetic data are as follows: 1HNMR (400MHz, CDCl 3 ): δ: 7.792 (d, J = 8.4 Hz, 4H); 7.350 (d, J = 8.4 Hz, 4H); 4.158 (t, J = 4.4 Hz, 4H); 3.702 ~ 3.567 (m, 4H); 2.435 (s, 6H);
[0030] (2) Add 175 g of Intermediate A obtained in step (1) to 400 ml of ethanol, and then add 68.6 g of sodium azide, raise the temperature to 80°C, and stir for 10 hours. After the reaction is complete, cool to room temperatur...
Embodiment 2
[0035] (1) Add 50g of diethylene glycol and 82g of pyridine to 500ml of acetonitrile, stir, weigh 198g of p-toluenesulfonyl chloride and dissolve in 250ml of acetonitrile, add dropwise to the reaction system under ice-water bath conditions, and then, at 30°C Reaction for 12h. TLC showed that the reaction was over, 400ml of water was added to wash, liquid separation, the organic phase was dried with anhydrous sodium sulfate, spin-dried, and then separated and purified by column chromatography to obtain 170g of Intermediate A, yield: 88%. The nuclear magnetic data are as follows: 1HNMR (400MHz, CDCl 3 ): δ: 7.782 (d, J=8.4 Hz, 4H); 7.356 (d, J=8.4 Hz, 4H); 4.156 (t, J=4.4 Hz, 4H); 3.710~3.568 (m, 4H); 2.434 (s, 6H);
[0036] (2) Add 170 g of Intermediate A obtained in step (1) to 400 ml of ethanol, and then add 66.6 g of sodium azide, raise the temperature to 80°C, and stir for 10 hours. After the reaction is complete, cool to room temperature, add 200 ml of water, extract with 5...
Embodiment 3
[0041] (1) Add 50g of diethylene glycol and 41g of sodium hydroxide to 500ml of dioxane, stir, weigh 225g of p-toluenesulfonyl chloride, dissolve in 250ml of acetonitrile dioxane, and add dropwise to the reaction system under ice-water bath conditions After dripping, react for 10h at 25°C. TLC indicated the completion of the reaction, 400 ml of water was added, extracted with dichloromethane, liquid separation, the organic phase was dried with anhydrous sodium sulfate, spin-dried, and then separated and purified by column chromatography to obtain 150 g of Intermediate A, yield: 77%. The nuclear magnetic data are as follows: 1HNMR (400MHz, CDCl 3 ): δ: 7.783 (d, J = 8.4 Hz, 4H); 7.366 (d, J = 8.4 Hz, 4H); 4.146 (t, J = 4.4 Hz, 4H); 3.713 ~ 3.560 (m, 4H); 2.435 (s, 6H);
[0042] (2) Add 150 g of Intermediate A obtained in step (1) to 400 ml of DMF, and then add 58.8 g of sodium azide, raise the temperature to 80°C, and stir for 10 hours. After the reaction is complete, cool to ro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com