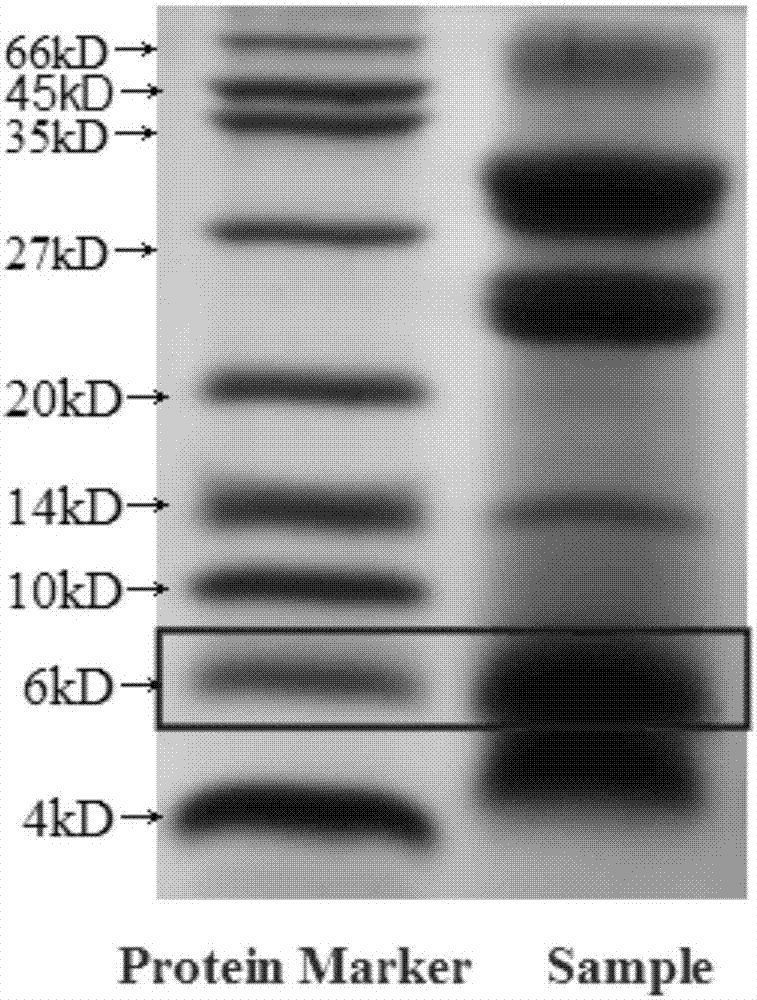
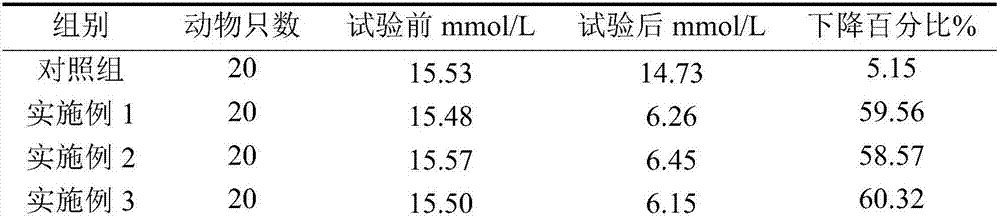
Momordica charantia polypeptide pharmaceutical composition for treating diabetes ulcer and preparation method thereof
A technology for diabetic ulcers and bitter gourd polypeptides, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems such as the inability to guarantee the stability and significance of product efficacy, the lack of quality controllability and content of key ingredients of raw materials, etc. Recover islet cell function, improve human immunity, and have a significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 provides a kind of bitter gourd polypeptide pharmaceutical composition for treating diabetic ulcer, and its specific steps are as follows:
[0061]Preparation of bitter gourd polypeptide extract: choose fresh bitter gourd with long growth period, greenish yellowish color and plump bitter gourd seeds as raw material, first wash the raw material of bitter gourd with tap water, and then wash with electrolytic high-concentration ozone water, the ozone concentration in the cleaning water is 15mg / L, water flow 300L / h, to reduce pesticide residues and microbial residues. Beat and crush the cleaned bitter gourd raw material into a liquid material passing through a 20-mesh screen; pour the beaten and crushed bitter gourd raw material into an ultrasonic assisted extraction tank, add 8 times the mass of purified water at 50°C, and use buffer salt to adjust the system pH=7, ultrasonic-assisted countercurrent extraction was carried out 3 times, 30 minutes each time. U...
Embodiment 2
[0064] Embodiment 2 provides a kind of bitter melon polypeptide pharmaceutical composition for the treatment of diabetic ulcers, and its specific steps are as follows:
[0065] Preparation of balsam pear polypeptide protein extract: select fresh balsam pear with long growth period, greenish yellowish color and plump balsam pear seeds as raw material, wash the balsam pear raw material with tap water first, and then wash with electrolytic high-concentration ozone water, the ozone concentration in water is 20mg / L, the water flow rate is 450L / h to reduce pesticide residues and microbial residues. Beating and crushing the washed bitter gourd raw material into a liquid material passing through a 20-mesh sieve. Pour the beaten and crushed bitter gourd raw materials into an ultrasonic-assisted extraction tank, add 5 times the mass of purified water at 38°C, use buffer salt to adjust the pH of the system to 6.7, and perform ultrasonic-assisted countercurrent extraction twice, each tim...
Embodiment 3
[0068] Embodiment 3 provides a kind of bitter gourd polypeptide pharmaceutical composition for treating diabetic ulcer, and its specific steps are as follows:
[0069] Preparation of bitter gourd polypeptide protein extract: select dried bitter gourd or sliced bitter gourd with greenish yellowish color and plump flesh as raw material, and crush the raw material of bitter gourd into coarse powder passing through a 20-mesh sieve. Pour the pulverized bitter melon raw material into an ultrasonic-assisted extraction tank, add 10 times the mass of purified water at 52°C, use buffer salt to adjust the pH of the system to 6.8, and conduct ultrasonic-assisted countercurrent extraction twice, each time for 45 minutes. Use a high-speed tubular centrifuge for centrifugation, and remove the slag to obtain a centrate. The centrifugate was filtered through a ceramic microfiltration membrane system with a molecular weight cut-off of 0.2 μm, the operating temperature was 35° C., and the pres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap