Blood sugar decreasing polypeptide-k compound capsule and preparation method thereof
A bitter gourd polypeptide, hypoglycemic technology, applied in the directions of capsule delivery, pharmaceutical formulations, peptide/protein components, etc., can solve the problem that the stability and significance of product efficacy cannot be guaranteed, the quality controllability of components and their content, and the preparation time period cannot be guaranteed. It can ensure stability and significance, eliminate diabetic complications, and achieve high absorption and utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] Further, the preparation method of this bitter gourd polypeptide extract comprises:
[0027] a. The bitter gourd raw material is extracted with purified water, and centrifuged to remove slag to obtain a centrate.
[0028] Optionally, choose fresh bitter gourd or dried bitter gourd and bitter gourd seeds as bitter gourd raw materials. The cleaning method is: firstly wash the raw bitter gourd raw materials with tap water, and then wash them with electrolytic high-concentration ozone water. The ozone concentration in the cleaning water can reach 10-20 mg / L , The water flow rate is 150-450L / h, which is used to further remove pesticide residues, microbial residues, etc.
[0029] Further, the method for extracting bitter gourd raw materials with purified water includes: pouring the crushed bitter gourd raw materials into an ultrasonic assisted extraction tank, adding 5 to 10 times the mass of purified water at 38 to 52°C, and adjusting the system by using buffer salts pH = 6...
Embodiment 1
[0052] This embodiment provides a hypoglycemic bitter gourd polypeptide compound capsule, the bitter gourd polypeptide compound capsule includes:
[0053] Bitter gourd polypeptide extract 4Kg, ginsenoside extract 3Kg, pueraria flavone extract 2Kg, astragalus polysaccharide extract 2Kg, trace element chromium 0.7‰.
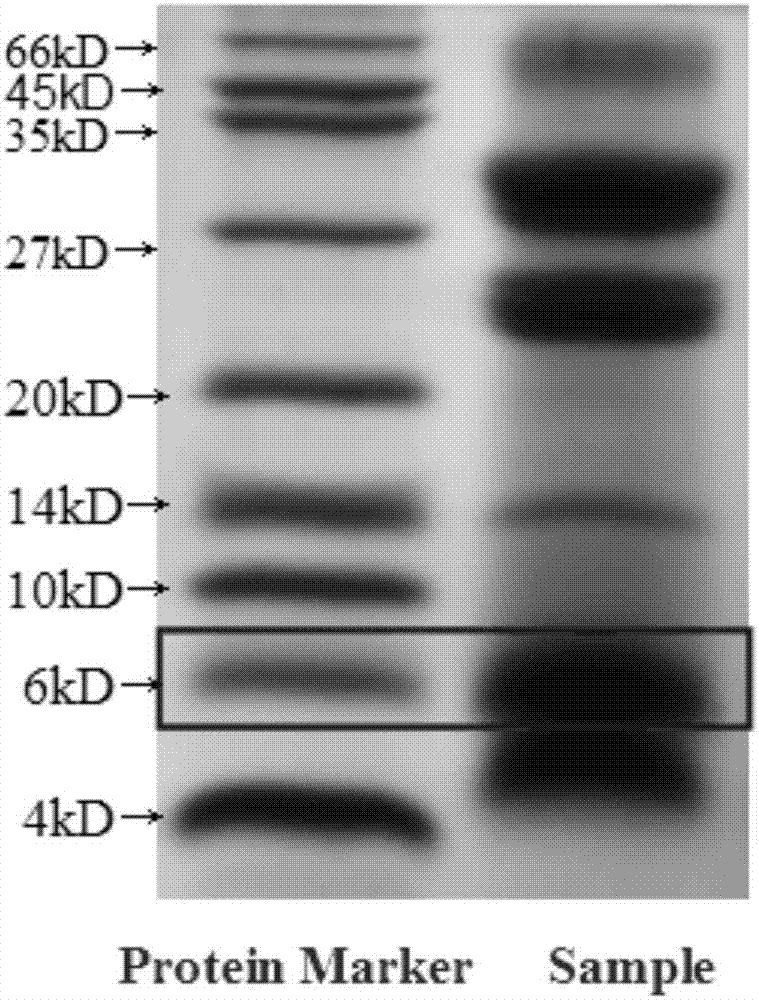
[0054] Among them, the content of bitter gourd polypeptide in the bitter gourd polypeptide extract is 28%, wherein the content of the bitter gourd polypeptide with a molecular weight of 5.5-7kDa accounts for 22%; the content of ginsenoside in the ginsenoside extract is 25%; The content of flavonoids is 72%, the content of puerarin in the total flavonoids is 48%; the content of astragalus polysaccharide in the extract of astragalus polysaccharide is 21%.
[0055]The preparation method of the bitter gourd polypeptide capsule comprises: combining bitter gourd polypeptide extract, ginsenoside extract, kudzu root extract, astragalus polysaccharide extract and trace elem...
Embodiment 2
[0057] This embodiment provides a bitter gourd polypeptide capsule with hypoglycemic effect, the bitter gourd polypeptide capsule includes:
[0058] Bitter gourd polypeptide extract 3Kg, ginsenoside extract 4Kg, kudzu root extract 1Kg, astragalus polysaccharide extract 3Kg, trace element chromium 0.6‰.
[0059] Among them, the content of bitter gourd polypeptide in the bitter gourd polypeptide extract is 31%, and the content of molecular weight is 5.5~7kDa accounts for 18%; The content of ginsenoside in the ginsenoside extract is 15%; The content of puerarin in the total flavonoids is 45%; the content of astragalus polysaccharide in the extract of astragalus polysaccharide is 15%.
[0060] The preparation method of the bitter gourd polypeptide capsule is consistent with that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com