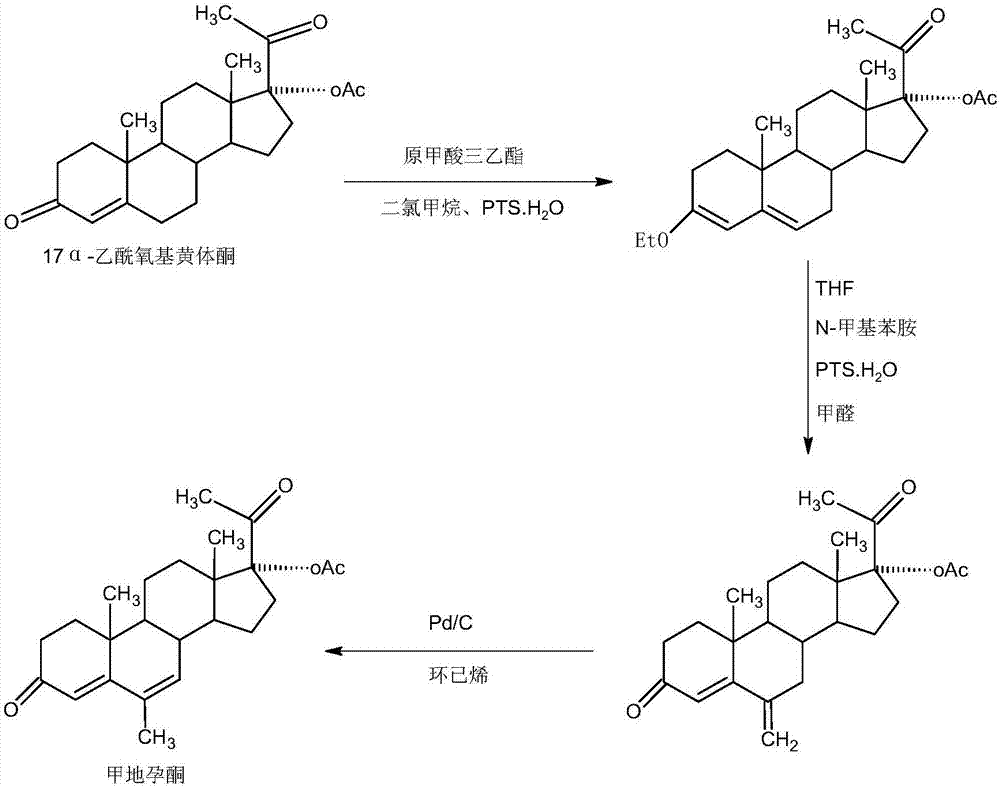
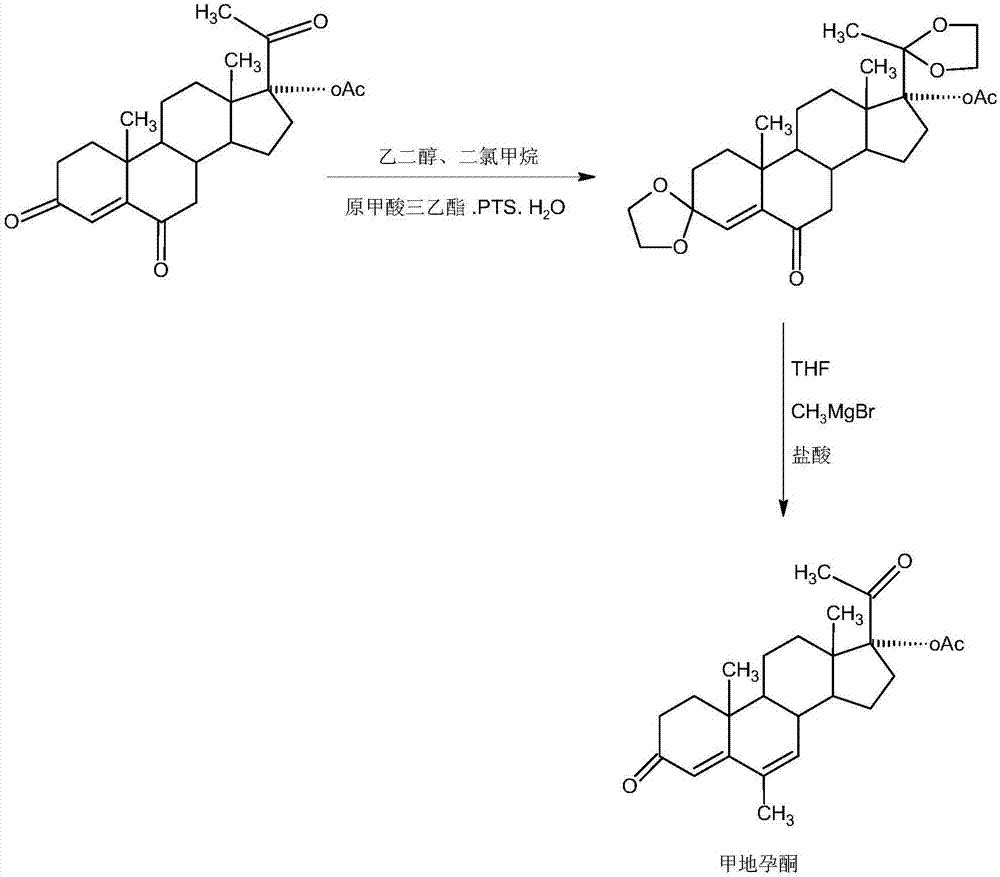
Megestrol acetate preparation method
A megestrol and ketone-based technology is applied in the field of preparation of steroid hormone drugs, can solve the problems of complex process operation, many impurities in the composition, and high production cost, and achieves simple and convenient process operation, high synthesis yield and production cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A. Preparation of diketals
[0022] In a 1000ml three-necked flask, add 100g 6 ketone base, 600ml dichloromethane, 50g ethylene glycol, 80ml triethyl orthoformate, 2g p-toluenesulfonic acid, and stir the reaction at 25~30 ℃ for 12~16 hours, TLC Detect the end point of the reaction, after the reaction is completed, add 3 ml of pyridine, stir for 20-25 minutes to neutralize the acid, then concentrate under reduced pressure, recover 90-95% of dichloromethane, then add 600 ml of tap water, stir and crystallize at 10-15 ° C for 3 -4 hours, centrifuged, washed with water to get the crude diketal product, the crude product was directly added to 800ml of 50% alcohol, refluxed for 1-1.5 hours, then steamed out about 400ml of alcohol at atmospheric pressure, and then cooled the system to -5-0 ℃, stirring for crystallization for 2-3 hours, suction filtration, washing with a small amount of ethanol, and drying the filter cake below 70 ℃ to obtain 118.2 g of diketal, HPLC content 99...
Embodiment 2
[0026] A. Preparation of diketals
[0027] In a 1000ml three-necked flask, add 100g of 6-ketone base, 600ml of toluene, 50g of ethylene glycol, 80ml of triethyl orthoformate, 2g of 98% sulfuric acid, keep the temperature at 25~30°C and stir the reaction for 12~16 hours, TLC detection At the end of the reaction, add 3ml of pyridine after the reaction, stir for 20-25 minutes to neutralize the acid, then concentrate under reduced pressure, recover 90-95% toluene, then add 600ml of tap water, stir and crystallize at 10-15°C for 3-4 hours, Centrifuge and wash with water to obtain the crude diketal product. The crude product is directly added to 800ml of 50% alcohol, first refluxed for 1-1.5 hours, then steamed out about 400ml of alcohol at normal pressure, and then the system is cooled to -5-0°C, The crystallization was stirred for 2 to 3 hours, suction filtered, washed with a small amount of ethanol, and the filter cake was dried below 70° C. to obtain 117.4 g of diketal, the HPLC...
Embodiment 3
[0031] A. Preparation of diketals
[0032] In a 1000ml three-necked flask, add 100g 6 ketone base, 600ml dichloromethane, 50g ethylene glycol, 80ml triethyl orthoformate, 10g ethanol solution of hydrochloric acid, keep the temperature at 25~30 ℃ and stir to react for 12~16 After the reaction is completed, add 3ml of pyridine, stir for 20-25 minutes to neutralize the acid, then concentrate under reduced pressure, recover 90-95% of dichloromethane, then add 600ml of tap water, stir at 10-15°C Crystallize for 3-4 hours, centrifuge, wash with water to obtain the crude diketal product, which is directly added to 800ml of 50% alcohol, refluxed for 1-1.5 hours, then steamed out about 400ml of alcohol at normal pressure, and then the system is cooled At -5-0°C, stirring for crystallization for 2 to 3 hours, suction filtration, washing with a small amount of ethanol, and drying the filter cake below 70°C to obtain 116.8 g of diketal, HPLC content of 99.6%, and weight yield of 116.8%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com