Manganese oxide material and preparation method thereof
A technology of manganese oxides and compounds, applied in the field of catalytic materials and environmental protection, can solve the problems of short life, difficult regeneration, and inability to remove pollutants at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
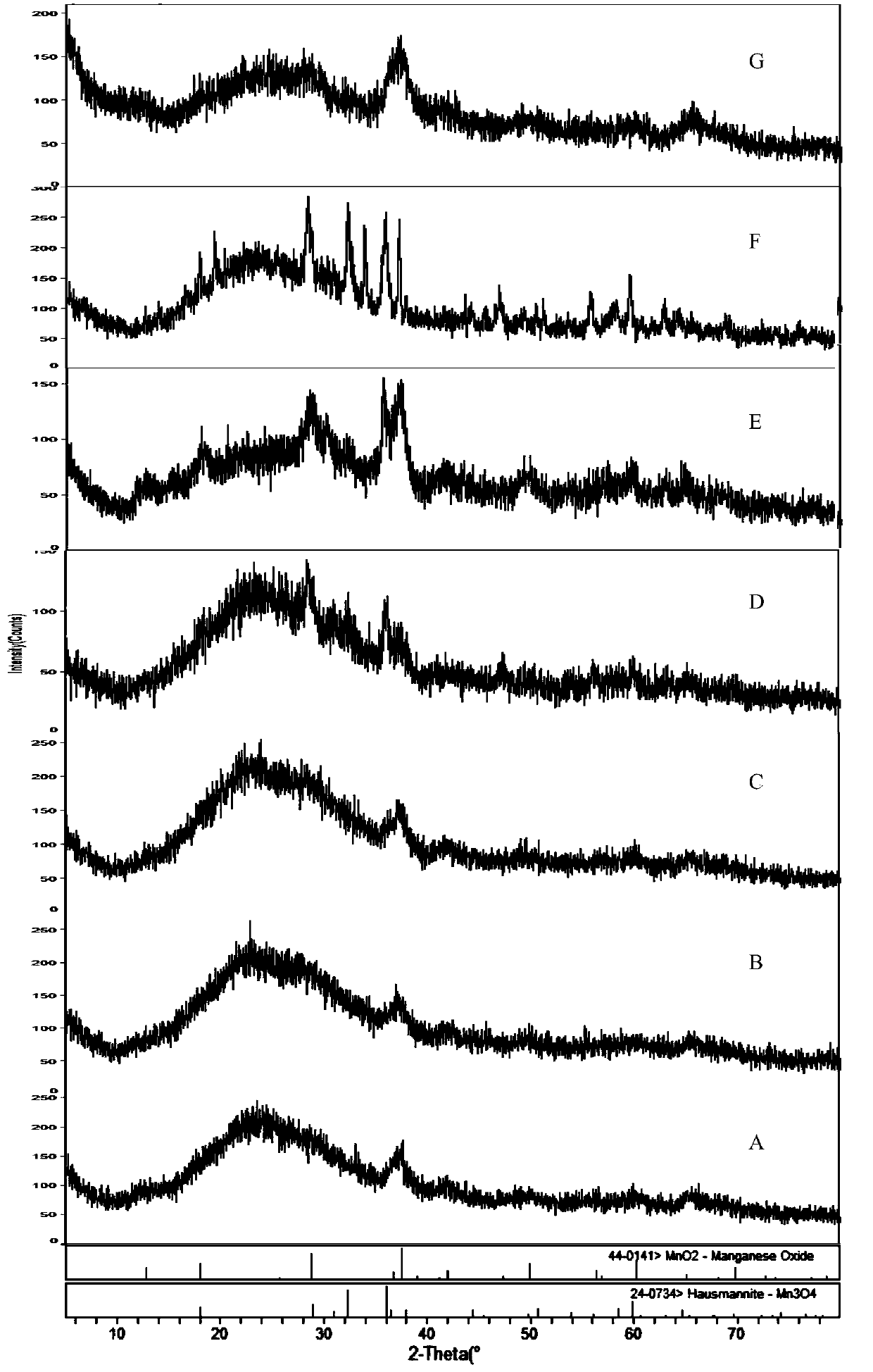
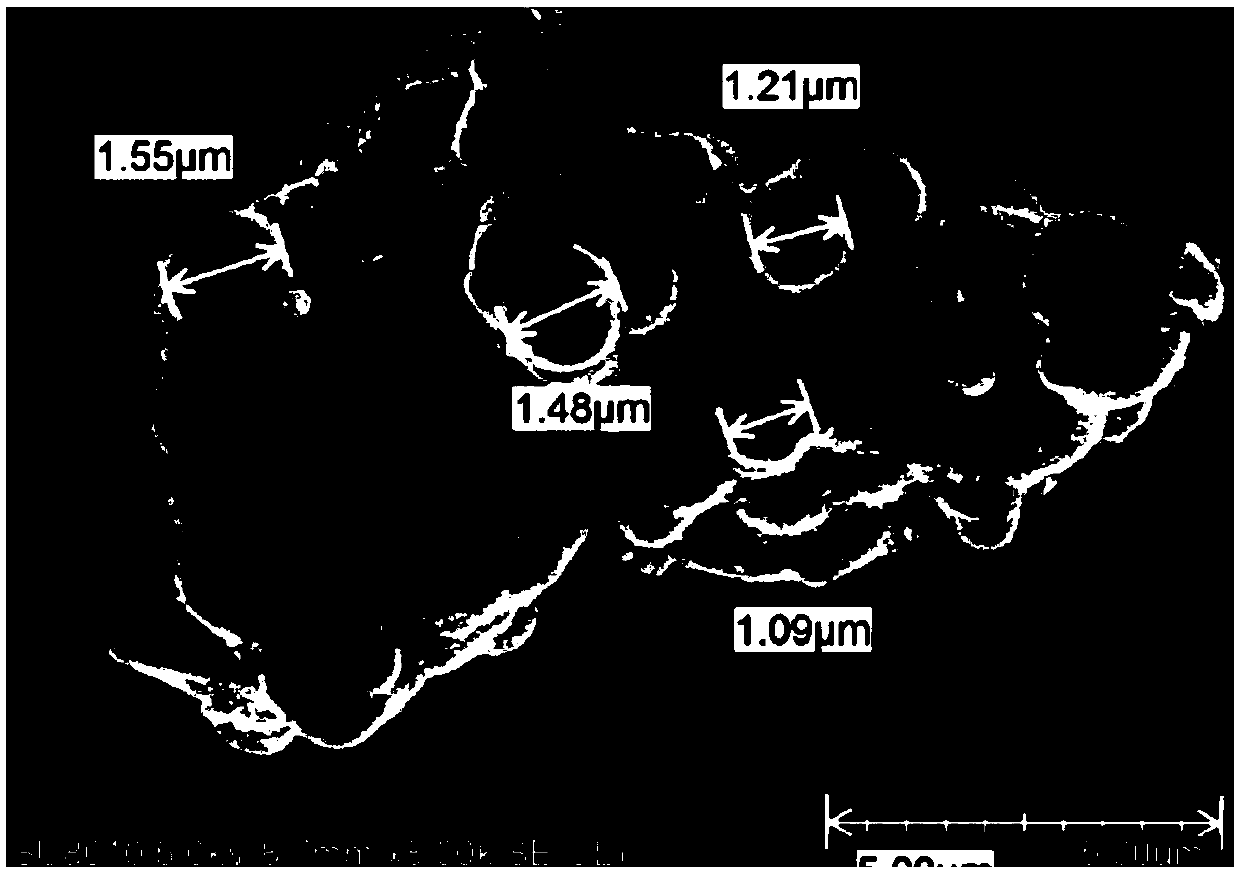
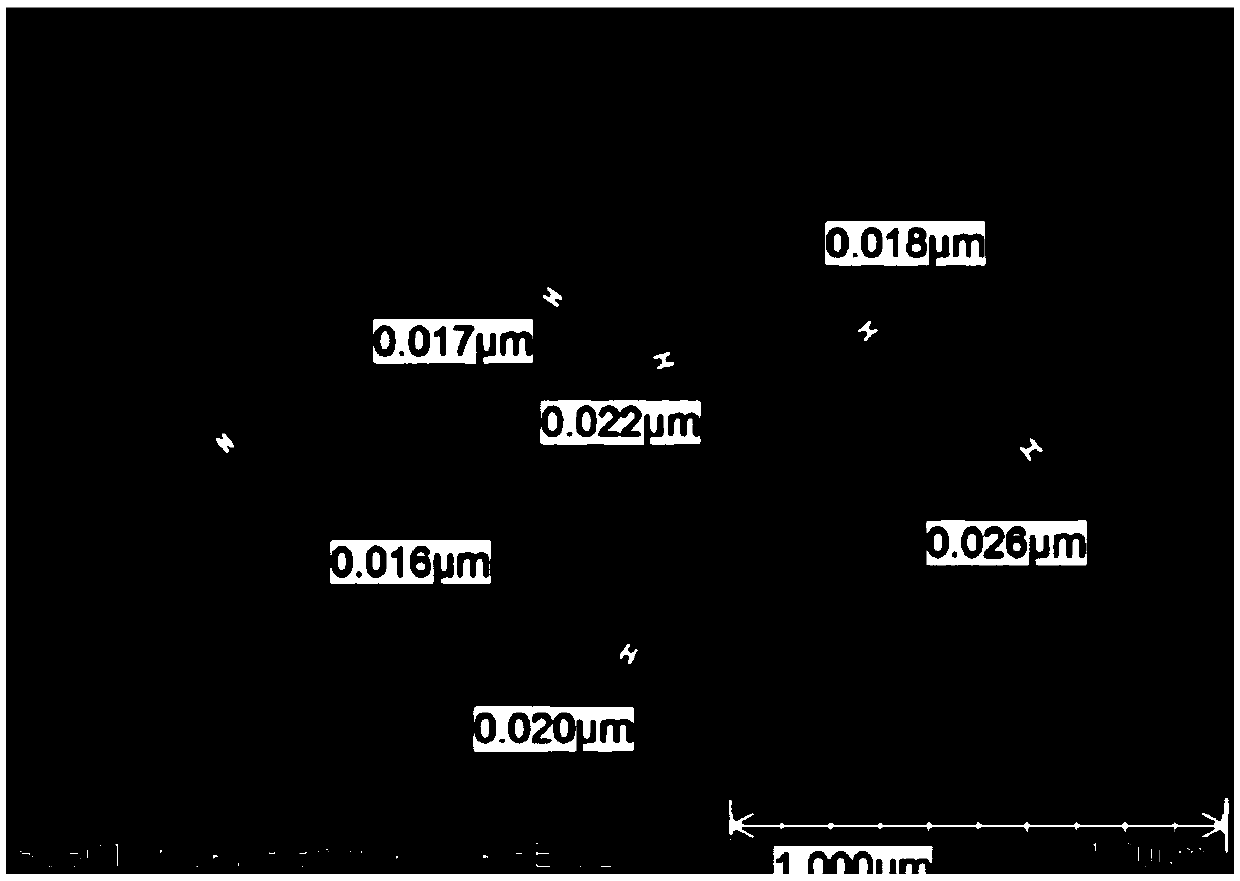
[0096] see figure 1 , figure 2 , image 3 , Figure 4 , Figure 5 , Figure 6 , Figure 7 . With 202.8 parts (weight, the same below) MnSO 4 ·H 2 O was dissolved in deionized water, and 126.4 parts of KMnO 4 , adjusted with NaOH and kept the pH above 12, kept the temperature at 50°C, and stirred for 2h. Then add 142.2 parts of MnSO 4 H2O, control the pH to 8-9, and stir at 50°C for 2 hours to obtain precipitate A. Then adjust the SO with sulfuric acid 4 2- Concentration of about 1mol / L, stirring at 50°C for 2h, to obtain precipitate B. Add CuSO after washing and filtering 4 、LaCl 3 , CeCl 3 , adjusted to pH 7-8, stirred for 2 hours to obtain precipitate C, washed and filtered, and dried to obtain manganese oxide material.
[0097]The content of each metal element detected by ICP and the chemical composition of the surface of the manganese oxide material measured by electron probe analysis (EDS) are shown in Table 1 (when the contents of each percentage are no...
Embodiment 2
[0132] see Figure 8 , Figure 9 , Figure 11 .
[0133] 190.4 parts MnCl 2 Dissolve and add 54.2 parts of sodium chlorate while stirring at 45°C while keeping the pH to 3-5. Then adjust the pH to 7 with potassium carbonate, then add 135.2 parts of MnSO 4 ·H 2 O, keep the temperature at 40 ° C and stir for 2.5h to obtain precipitate B. Precipitate B was washed, filtered and dried to obtain manganese oxide material I. After washing and filtering the precipitate B, add CuSO 4 The solution was stirred, adjusted to pH 8-9 with NaOH, washed after 2 hours, filtered and dried to obtain manganese oxide material II.
[0134] The contents of each metal element in manganese oxide materials Ⅰ and Ⅱ detected by ICP are shown in Table 6. After doping with copper, the active sites and vacancy defects are increased, showing better catalytic activity.
[0135] Y(Mn 2+ ) / Y(Mn 4+ ) = 0.18. mn 2+ After being adsorbed by manganese dioxide, a part is oxidized to Mn due to surface auto...
Embodiment 3
[0157] see Figure 10 , Figure 12 .
[0158] 336.4 parts of MnCl 2 Dissolve in deionized water. At 20°C, with NH 3 ·H 2 O to adjust the pH to 10, while stirring, hydrogen peroxide was added dropwise to produce substantially no foam. Then with 338.4 parts of MnSO 4 ·H 2 O mixed and adjusted the pH of the system to 7.5, stirred at room temperature for 8h. Then adjust SO with sulfuric acid and ammonium sulfate 4 2- The concentration is about 0.1mol / L, stirred at room temperature for 3 hours, washed, filtered and dried to obtain manganese oxide material.
[0159] The use of sulfuric acid when adjusting the sulfate concentration can reduce or eliminate the impurity Mn(OH) 2 . Because Mn(OH) 2 Easy hydration, resulting in high water absorption of manganese oxides, affecting the performance of manganese oxides. Using sulfuric acid and ammonium sulfate to adjust the sulfate concentration can form a buffer solution, which is beneficial to the stability of the system.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com