Preparation method of conjugated diene compound
A technology of conjugated dienes and compounds, which is applied in fine chemicals and related chemical fields, can solve problems such as high reaction temperature, and achieve the effect of easy-to-obtain, cheap raw materials and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
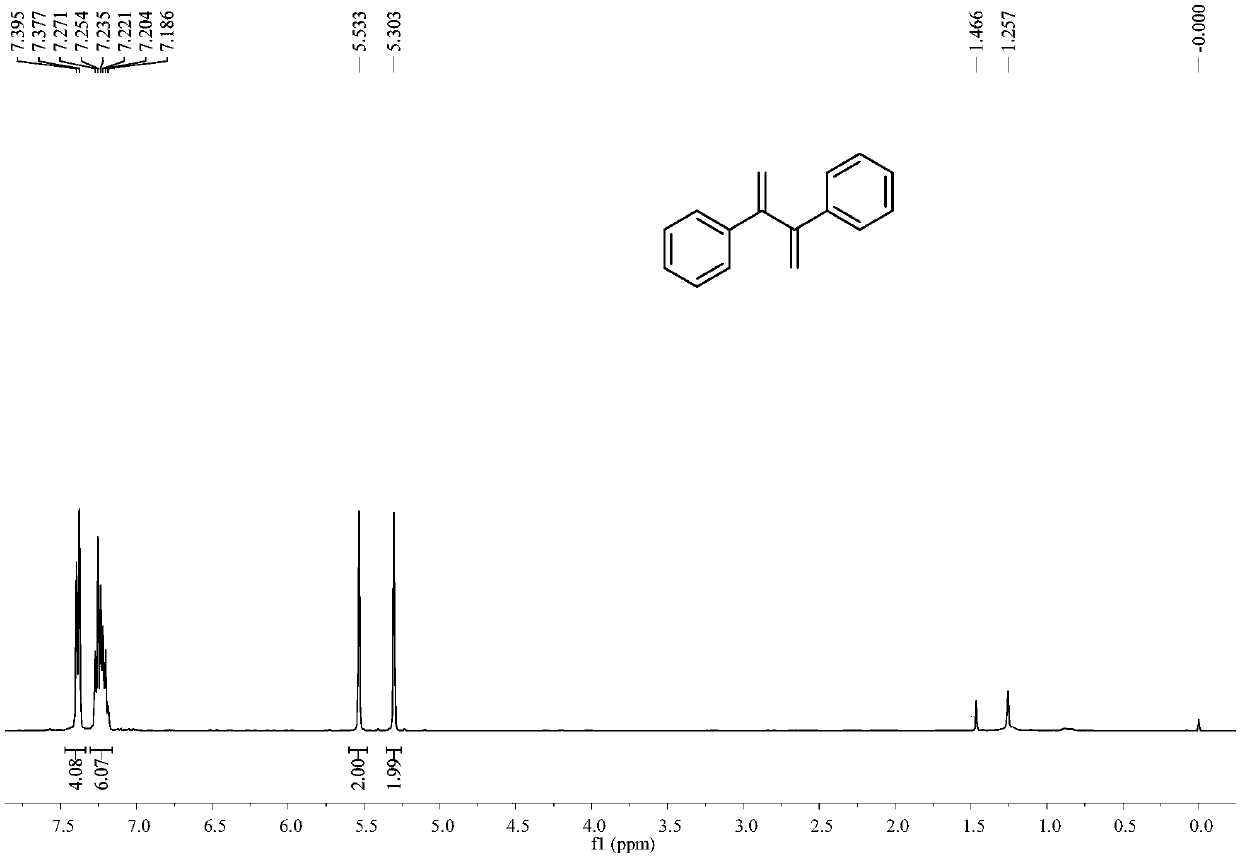
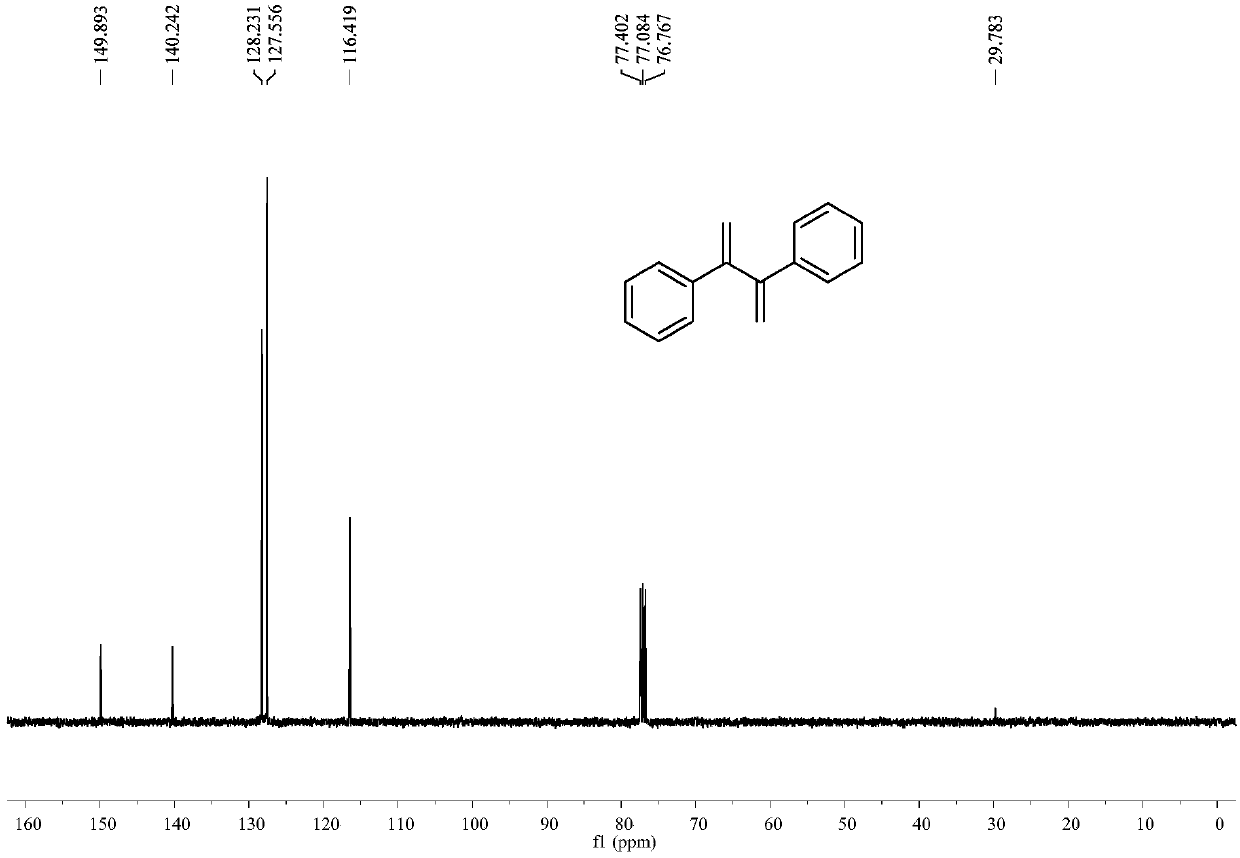
[0037] Example 1: Synthesis of 2,3-diphenyl-1,3-dibutene
[0038] In a 25mL reactor, add p-toluenesulfonic acid (0.029g, 0.15mmol), pivalic acid (0.087g, 0.85mmol), zinc powder (0.033g, 0.5mmol), tetrakis(triphenylphosphine) palladium (0.023 g, 0.02mmol), nitrogen replacement 3 times, add anhydrous dichloromethane 3mL, add phenylacetylene (0.051g, 0.5mmol) under stirring, stir at 25°C for 24h. Column chromatography (silica gel, 200-300 mesh; developer, petroleum ether) obtained 0.038 g of 2,3-diphenyl-1,3-dibutene with a yield of 73%.
[0039] 2,3-Diphenyl-1,3-dibutene
[0040] colorless crystals; 1 H NMR (CDCl 3 ,400MHz)δ7.38-7.40(m,4H),7.19-7.27(m,6H),5.53(s,2H),5.30(s,2H); 13 C NMR (CDCl 3 ,100MHz)δ149.9,140.2,128.2,127.6,116.4ppm; MS(EI)m / z=207,206,191,178,128,115,91.
Embodiment 2
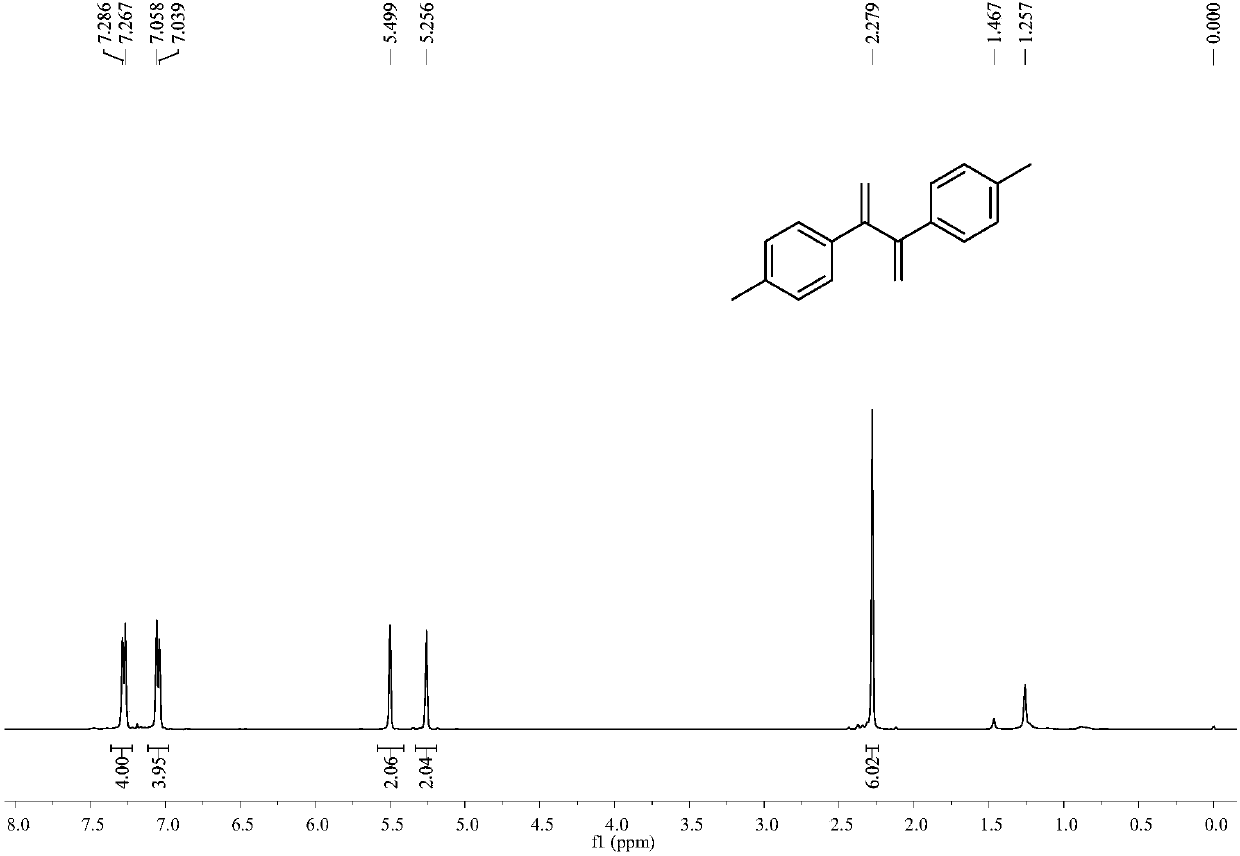
[0041] Example 2: Synthesis of 2,3-bis(4-methylphenyl)-1,3-dibutene
[0042] The operation was the same as in Example 1, and 0.049 g of 2,3-bis(4-methylphenyl)-1,3-dibutene was obtained by reacting 4-methylphenylacetylene, with a yield of 83%.
[0043] 2,3-bis(4-methylphenyl)-1,3-dibutene
[0044] white solid; 1 H NMR (CDCl 3 ,400MHz)δ7.28(d,J=8.0Hz,4H),7.05(d,J=8.0Hz,4H),5.50(s,2H),5.26(s,2H),2.28(s,6H); 13 C NMR (CDCl 3 ,100MHz)δ149.8,137.4,137.2,128.9,127.3,115.4,21.2ppm; MS(EI)m / z=235,234,219,204,128,115,91
Embodiment 3
[0045] Example 3: Synthesis of 2,3-bis(4-methoxyphenyl)-1,3-dibutene
[0046] The operation was the same as in Example 1, and 0.060 g of 2,3-bis(4-methoxyphenyl)-1,3-dibutene was obtained from the reaction of 4-methoxyphenylacetylene, with a yield of 90%.
[0047] 2,3-bis(4-methoxyphenyl)-1,3-dibutene
[0048] white solid; 1 H NMR (CDCl 3 ,400MHz)δ7.31(d,J=8.0Hz,4H),6.78(d,J=8.0Hz,4H),5.47(s,2H),5.23(s,2H),3.74(s,6H); 13 C NMR (CDCl 3 ,100MHz)δ159.1,149.4,132.7,128.5,114.3,113.6,55.2ppm; MS(EI)m / z=267,266,251,235,121
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com