A kind of amino-substituted staurosporine compound and its preparation method and application
A technology for staurosporine and compounds, applied in the field of amino-substituted staurosporine compounds and their preparation, can solve problems such as poor selectivity, and achieve the effects of high inhibitory effect, good application prospect and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 1. Seed solution
[0038] The above Streptomyces was inoculated into 500mL Erlenmeyer flasks containing 250mL of liquid medium, each bottle containing 250mL of Gaoshi No. 1 liquid medium, and cultured in a shaker at 28°C at 180rpm for 4 days to obtain seed liquid.
[0039] 2. Fermentation
[0040] Inoculate the above-mentioned seed liquid into the rice fermentation medium (made by the following weight components: rice 40g, 25% sea salt water 60mL) with the amount of inoculating 8mL per bottle, and stop the fermentation after 60 days of static culture at 28°C.
[0041] 3. Rough extraction
[0042] Soak each bottle of solid fermented product with about 200 mL of EA (ethyl acetate) overnight, filter through three layers of gauze to remove mycelia, collect the filtrate, and concentrate under reduced pressure to obtain a crude extract (oily extract).
[0043] 4. Separation and purification
[0044] The above crude extract was extracted three times with an equal volume of ...
Embodiment 2
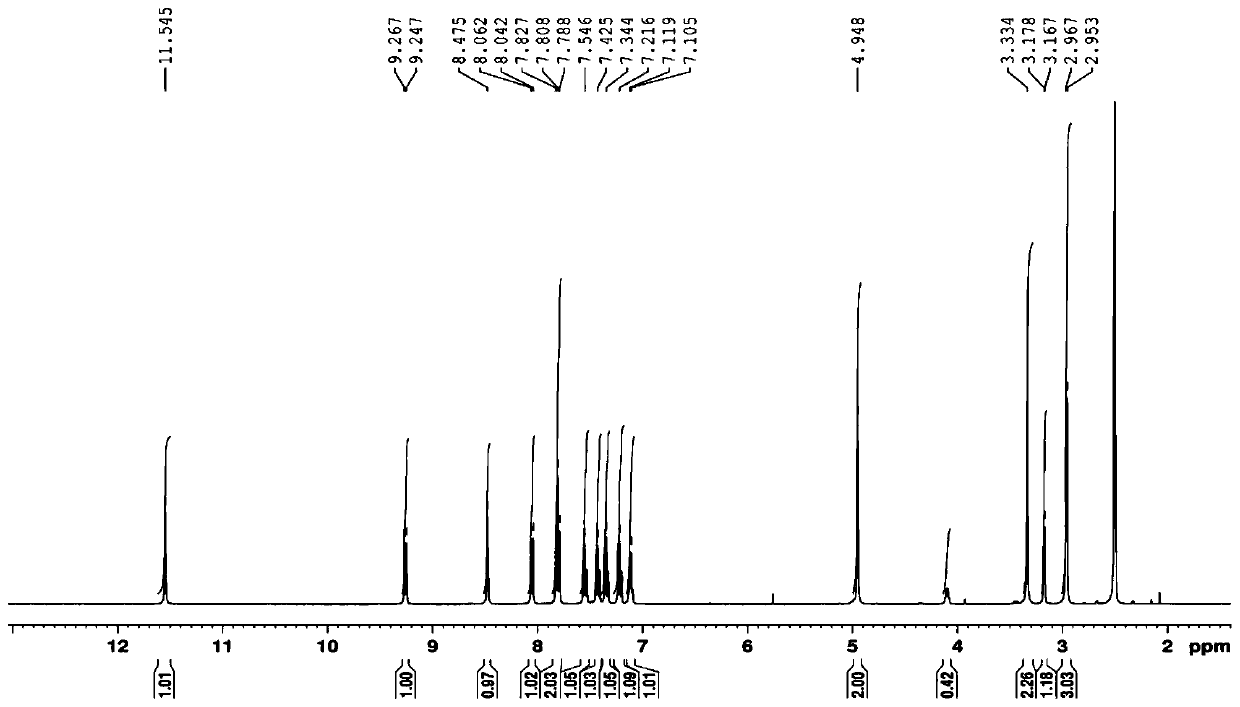
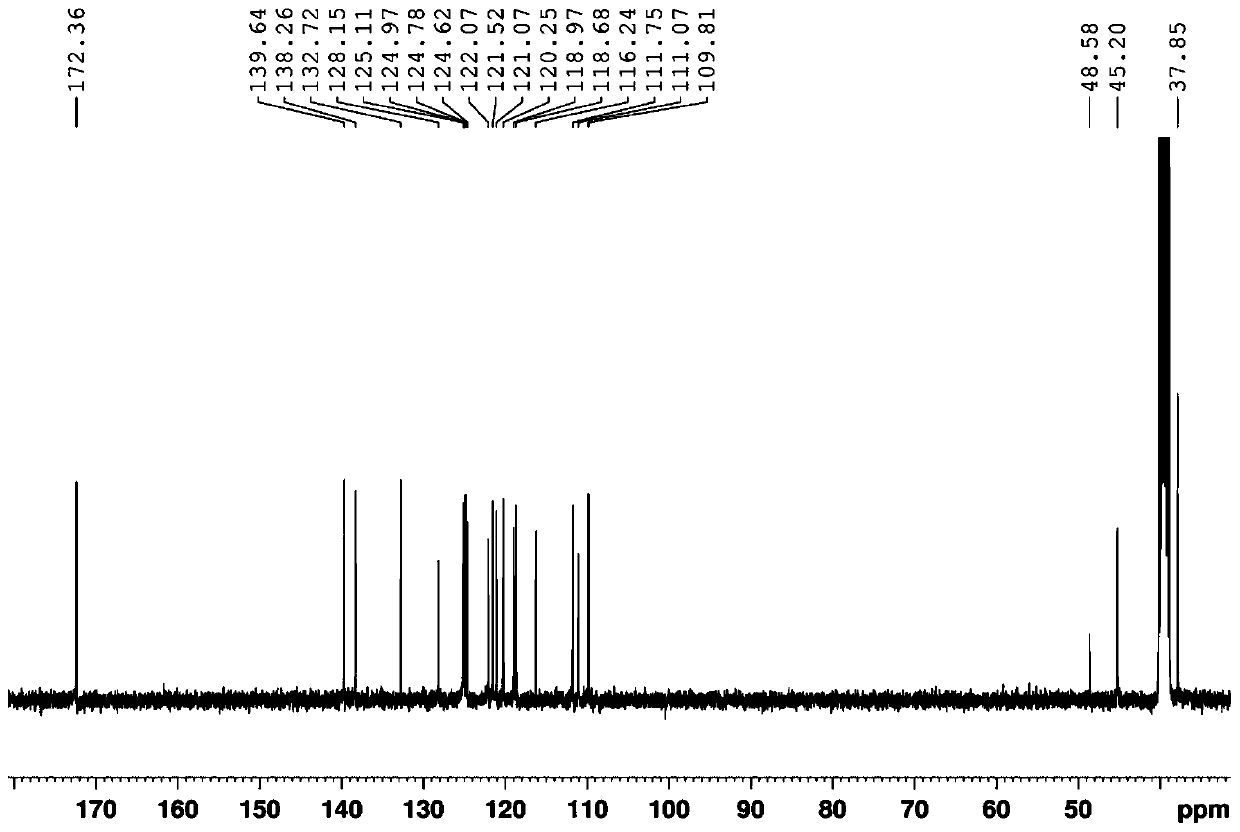
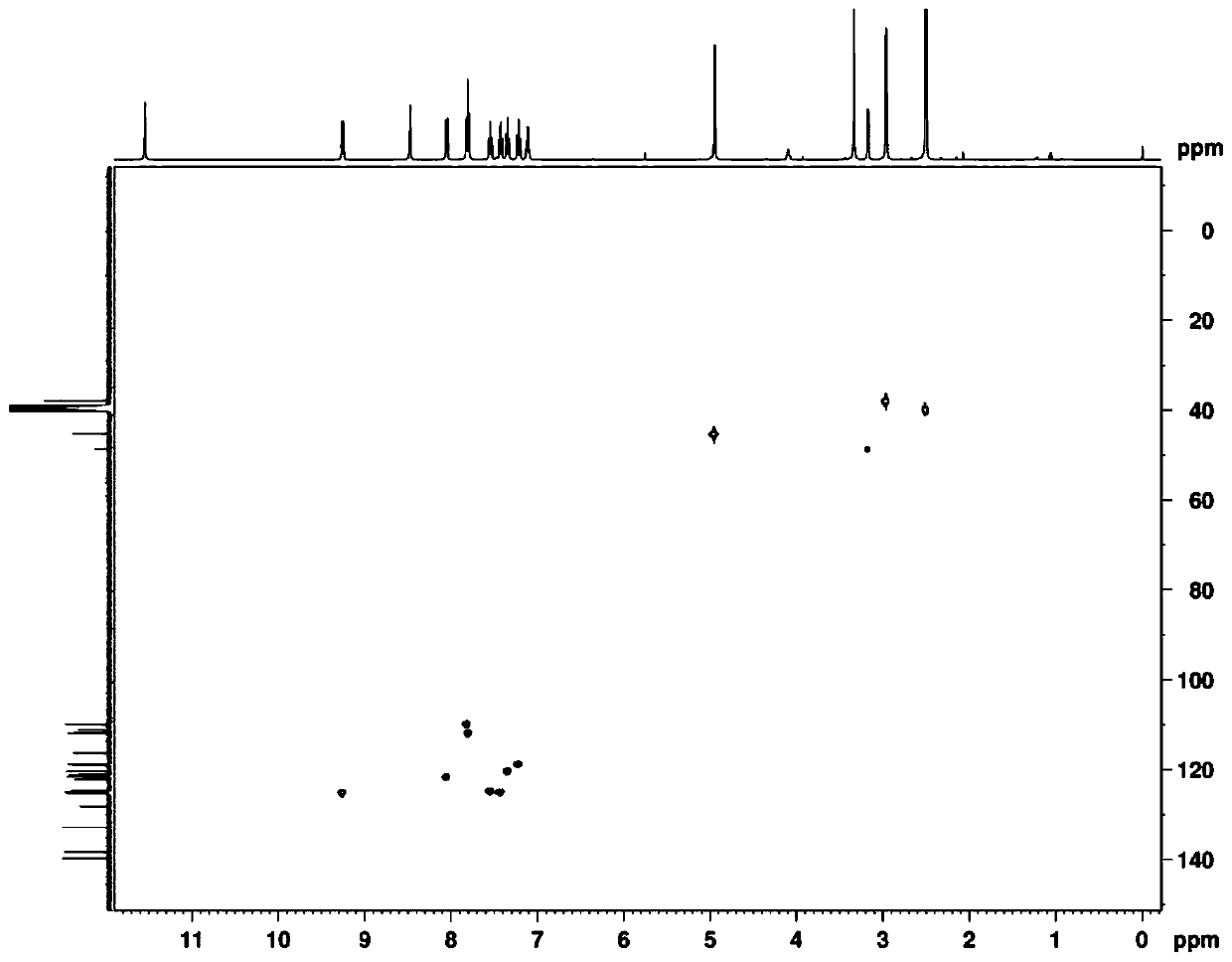
[0047]The new compound obtained in the present invention is a white amorphous powder, and the high-resolution mass spectrum provides [M+H] at HRESIMS m / z 341.1381 + peak, suggesting a molecular weight of 340, combined with 1 H-NMR, 13 C-NMR and HSQC spectral data ( figure 1 , 2 and 3) the presumed molecular formula is C 21 h 16 N 4 O, the degree of unsaturation is 16. exist 1 The downfield region of H-NMR shows 8 aromatic proton signals δ H 9.26 (1H, d, J = 8.0Hz), 8.05 (1H, d, J = 7.8Hz), 7.82 (1H, d, J = 7.8Hz), 7.80 (1H, d, J = 7.8Hz), 7.55 ( 1H, brt), 7.42(1H, br t), 7.34(1H, br t), 7.22(1H, br t); three active hydrogen signals δ H 11.54 (1H, s), 8.47 (1H, s), 7.10 (1H, q, J = 5.5Hz); there is a methylene signal 4.95 (2H, s) and a methyl signal δ in the high field H 2.95 (3H, d, J=5.5Hz). Compared with the reported K252c, it is found that this compound has one more methyl group and one active hydrogen signal. further analysis 1 H- 1 H COZY and HMBC spectra ...
Embodiment 3
[0049] Proliferation inhibition experiment of human prostate cancer cell line PC3.
[0050] Cells in the logarithmic growth phase were taken and configured as 5×10 4 cells / mL, spread in 96-well culture plate at 100 μL / well, CO 2 Cultivate in the incubator for 24 hours, take out the culture plate, add different concentrations of samples to be tested in each well, set 3 duplicate holes for each concentration, and place in CO 2 After continuing to culture in the incubator for 72 hours, take out the culture plate, discard the culture medium, add 100 μL of 10% trichloroacetic acid (TCA) pre-cooled in a refrigerator at 4°C to each well to fix it, let it stand for 5 minutes, and then move the culture plate to 4 °C refrigerator overnight. Pour off the fixative, wash each well 5 times with deionized water, spin dry, and air dry. Add 70 μl of SRB solution to each well, place at room temperature for 20 minutes, remove the supernatant, wash 5 times with 1% acetic acid, and air dry. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com