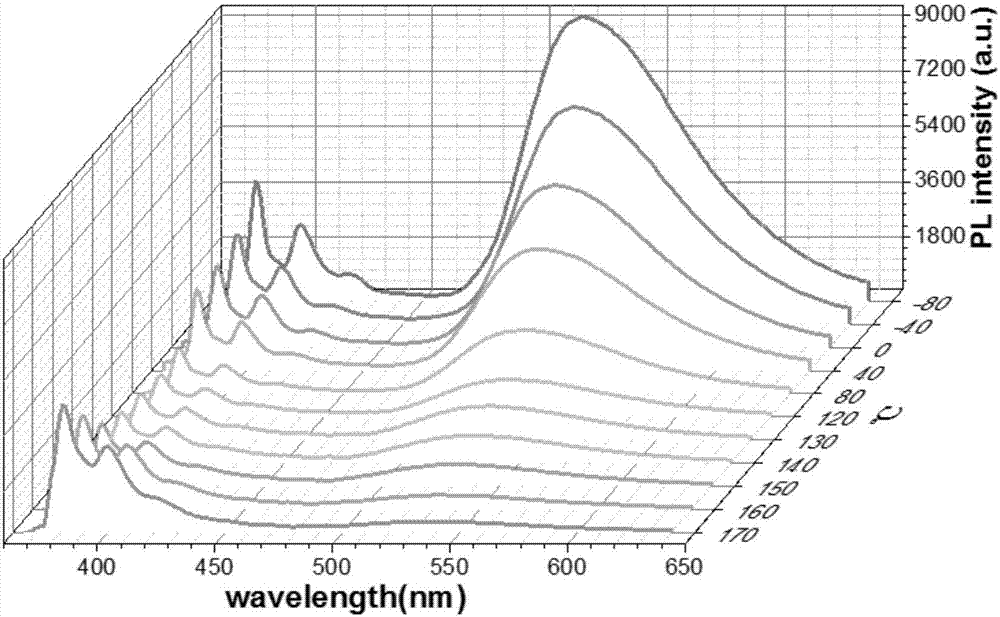
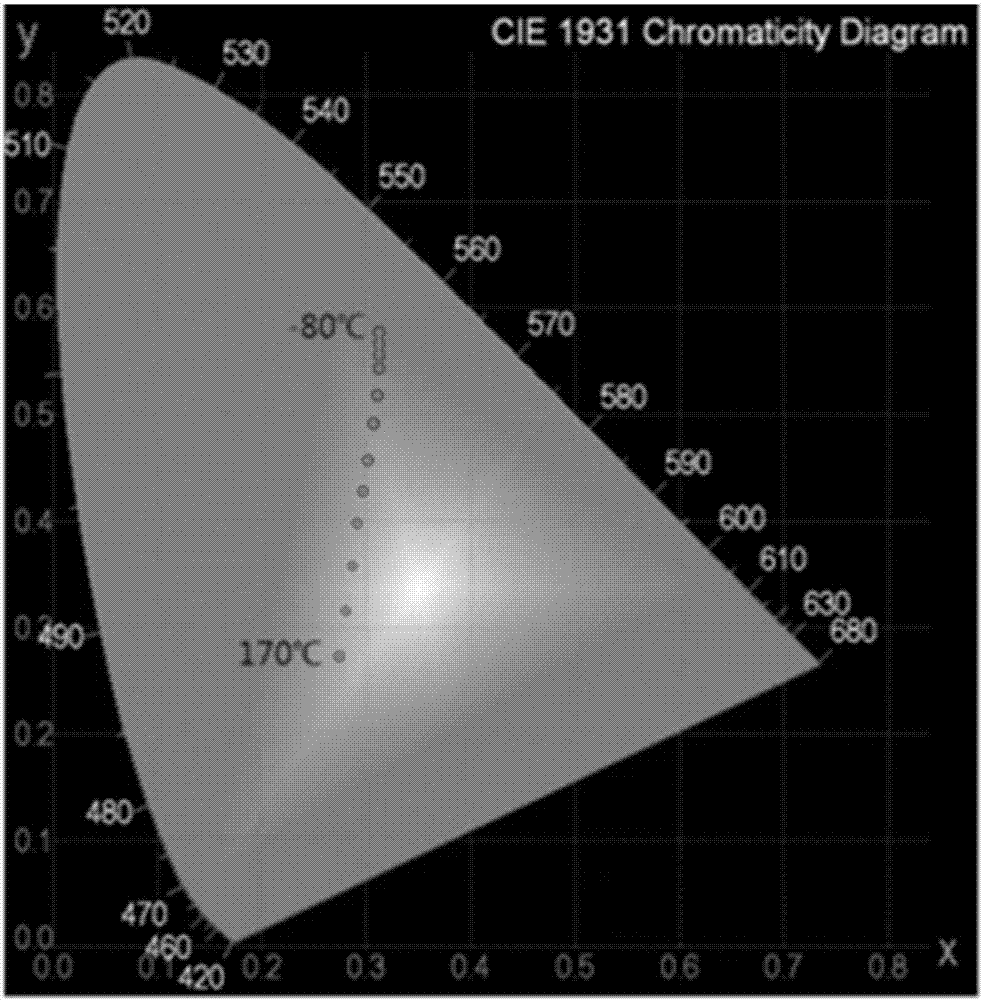
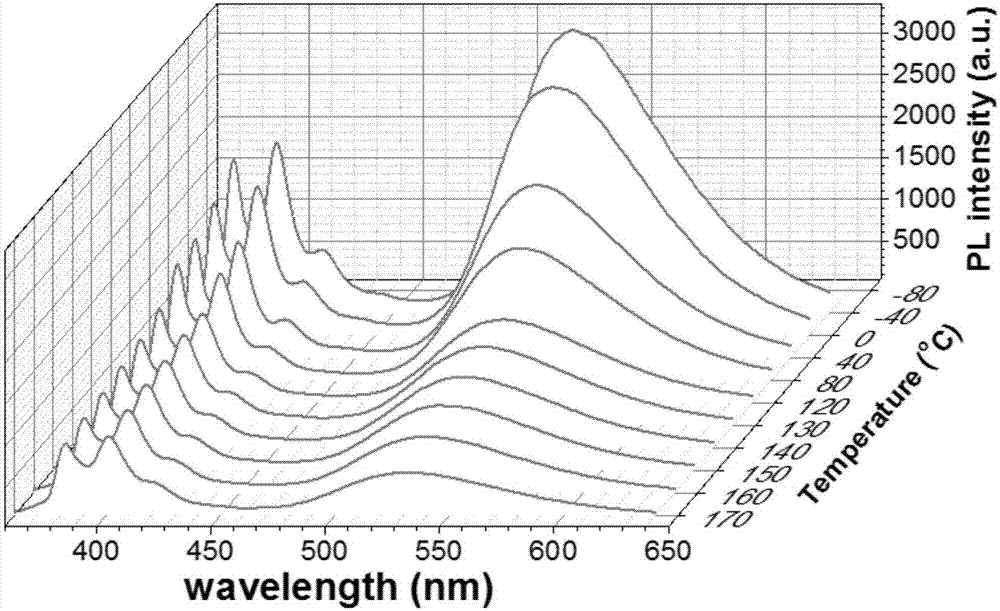
Dual-luminescence organic fluorescent temperature sensing film and preparation method thereof
A technology for sensing film and fluorescence temperature, applied in the field of dual-luminescence organic fluorescence temperature sensing film and preparation, and the field of organic fluorescence temperature sensing film, which can solve the problems of different emission colors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Embodiment 1: Preparation of triaryl phosphine oxide
[0109] Preparation of compound phenyl bis(1'-pyrene) phosphine oxide, the reaction scheme is as follows:
[0110]
[0111] Take a 50ml three-neck flask with a built-in magnet, and bake for 12 hours to remove water. The gas was extracted three times on the double-row pipe gas path. Under the protection of nitrogen, 1-bromopyrene (1g, 3.8mmol, 2equiv) was dissolved in dry ether, cooled in a dry ice acetone bath, and n-butyllithium was added at low temperature. (2.5ml, 3.8mmol, 2equiv), after stirring for 10-48h, remove the dry-ice acetone bath and heat up to normal temperature, then react for 0.5-3h. After the reaction, phenyl phosphorus dichloride (0.34 g, 1.9 mmol, 1 equiv) was added dropwise to the reaction system, and the reaction was continued for 0.5 to 6 h under nitrogen protection. After the reaction, extract the organic phase with dichloromethane, combine the organic phases, add anhydrous sodium sulfate,...
Embodiment 2
[0113] Embodiment 2: Preparation of large Stokes shift compound
[0114] Preparation of compound 2-(benzo[d]thiazol-2-yl)-4-bromophenol, the reaction scheme is as follows:
[0115]
[0116]A reaction device was set up with an iron stand, and 2-aminothiophenol (40mmol) and 5-aminosalicylic acid (40mmol) were added to a large amount of catalyst, and then the gas was ventilated three times. Then set the reaction temperature, turn on condensed water, heat to a high temperature in a nitrogen atmosphere, and reflux for 1 to 12 hours. After the reaction was complete, the reaction bottle was lifted, and after cooling, the mixture was dissolved in a large amount of ice-water mixture, placed in an ice-water bath, and then a prepared saturated sodium hydroxide (NaOH) aqueous solution was added thereto for neutralization. The neutralized solution was suction filtered. The solid extracted by suction filtration was extracted three times with ethyl acetate, the combined organic layers w...
Embodiment 3
[0123] Embodiment 3: prepare phenyl two (1 '-pyrene) phosphine oxide and 2-(benzo [d] thiazol-2-yl) -4- (pyrene-1-yl) phenol co-doped PMMA thermosensitive rigidity and Flexible Dual Emission Organic Fluorescent Temperature Sensing Film:
[0124] The triarylphosphoroxy blue light compound phenyl bis(1'-pyrene) phosphine oxide and the large Stokes shift yellow light compound 2-(benzo[d]thiazol-2-yl)-4-(pyrene-1-yl ) phenol was blended with dichloromethane solution at a mass ratio of 1:6, and then blended with PMMA at a mass ratio of 1 wt%, and dissolved in 30ml of dichloromethane to obtain a mixed solution. Spin-coat half of the mixed solution on a 2*2cm quartz sheet with a homogenizer to obtain a rigid double-luminescence organic fluorescent temperature sensing film, and spin-coat half of the mixed solution on a 2*2cm PET with a homogenizer to obtain a flexible dual-luminescence Organic fluorescent temperature sensing film.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com