A kind of aqueous electrolyte and aqueous metal ion battery
A metal ion and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve the problems of material dissolution, low capacity retention rate or Coulomb efficiency, achieve a large electrochemical window, inhibit hydrogen evolution/oxygen evolution, etc. The effect of reaction, good conduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Dimethyl sulfone, LiClO 4 (anhydrous) and H 2 O was mixed according to the molar ratio of 1:0.5:0.25, heated and stirred at 80°C, and allowed to cool to room temperature to obtain an aqueous electrolyte.
[0043] Then, the glassy carbon electrode was used as the working electrode, the platinum wire electrode was used as the counter electrode, and the silver-silver chloride electrode was used as the reference electrode, and a three-electrode system was used for testing.
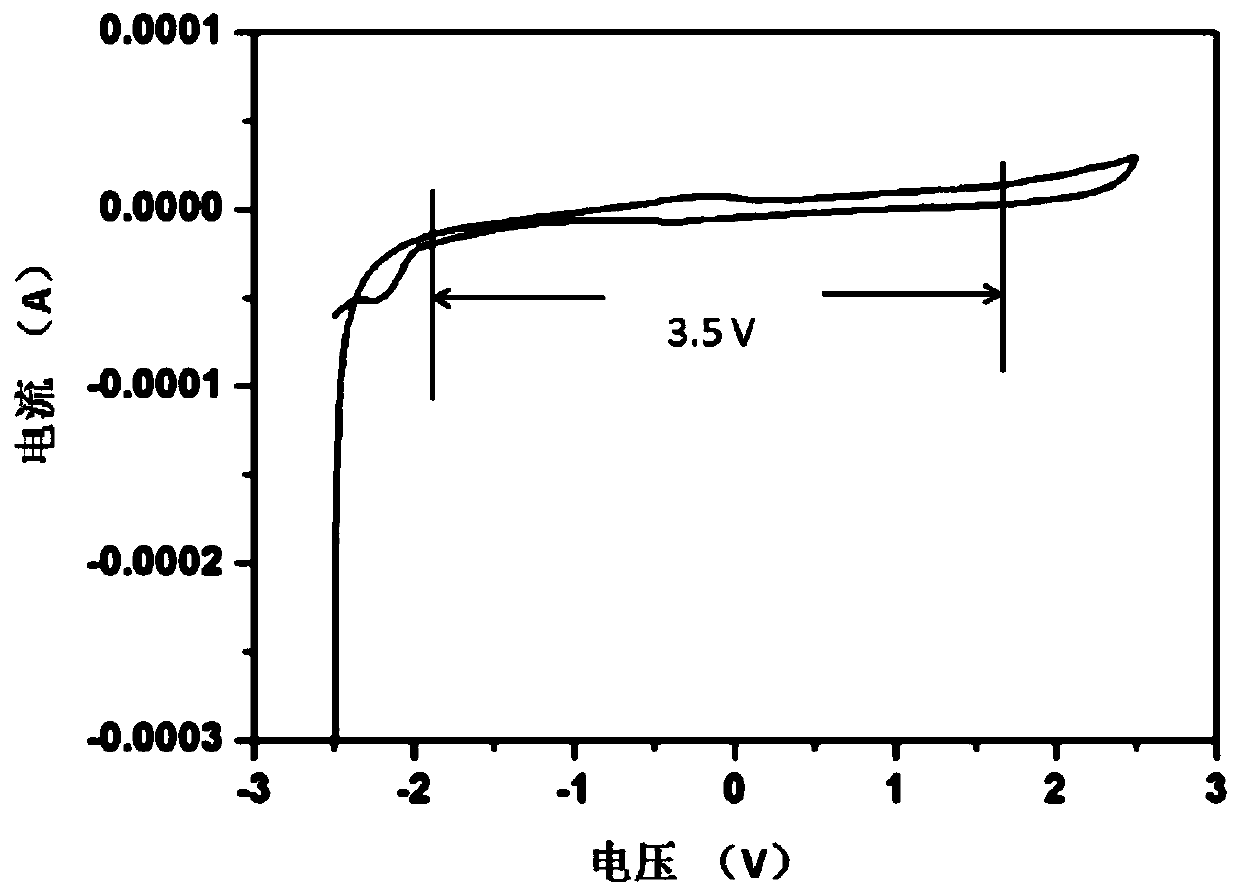
[0044] The above-mentioned three-electrode system was subjected to a cyclic voltammetry test on a strong transmission (UK strong transmission) with a voltage range of -2.5 volts to 2.5 volts and a scan rate of 20 mV / S.
[0045] figure 1 For the cyclic voltammetry curve that the embodiment of the present invention 1 obtains, by figure 1 It can be seen that the voltage window of the electrolyte solution in Example 1 of the present invention reaches 3.5V.
Embodiment 2
[0047] Dimethyl sulfone, LiClO 4 .3H 2 O, LiClO 4 (Anhydrous) Mix according to the molar ratio of 2:0.33:0.67, heat and stir at 80°C, and let it cool to room temperature to obtain an aqueous electrolyte.
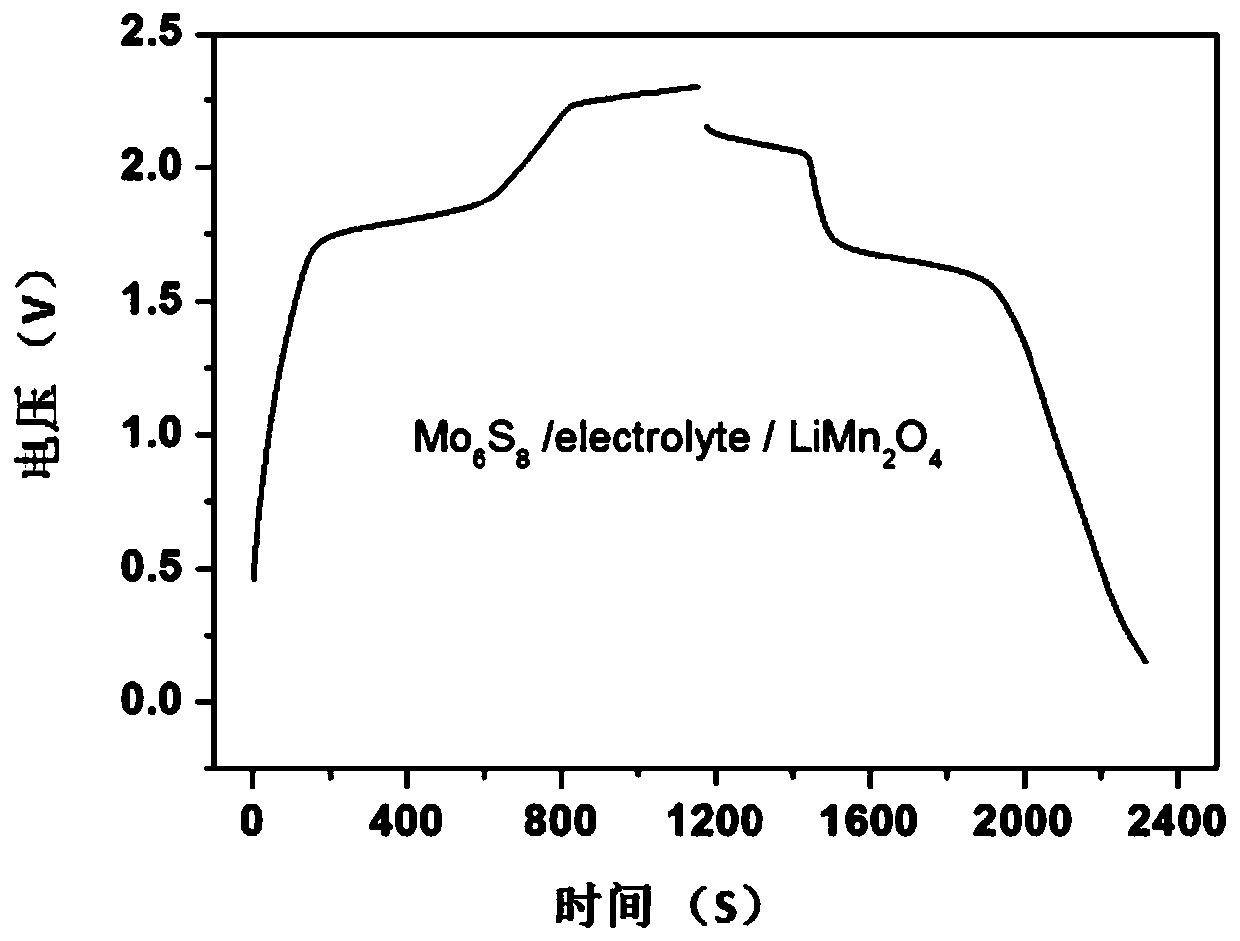
[0048] Then, with Mo 6 S 8 as the negative electrode, LiMn 2 o 4 As the positive electrode, a full battery is assembled without a separator. Wherein, the positive and negative electrode slurries are prepared according to m (active material): m (polyvinylidene fluoride, PVDF): m (acetylene black) = 75:10:15, and the prepared slurry is applied to the Ti grid , the mass of the positive electrode is about 5-8mg, and the mass of the negative electrode is 30%-40% greater than that of the positive electrode. Put the coated pole pieces into an oven at 80°C, and bake in an air atmosphere for 13 hours.
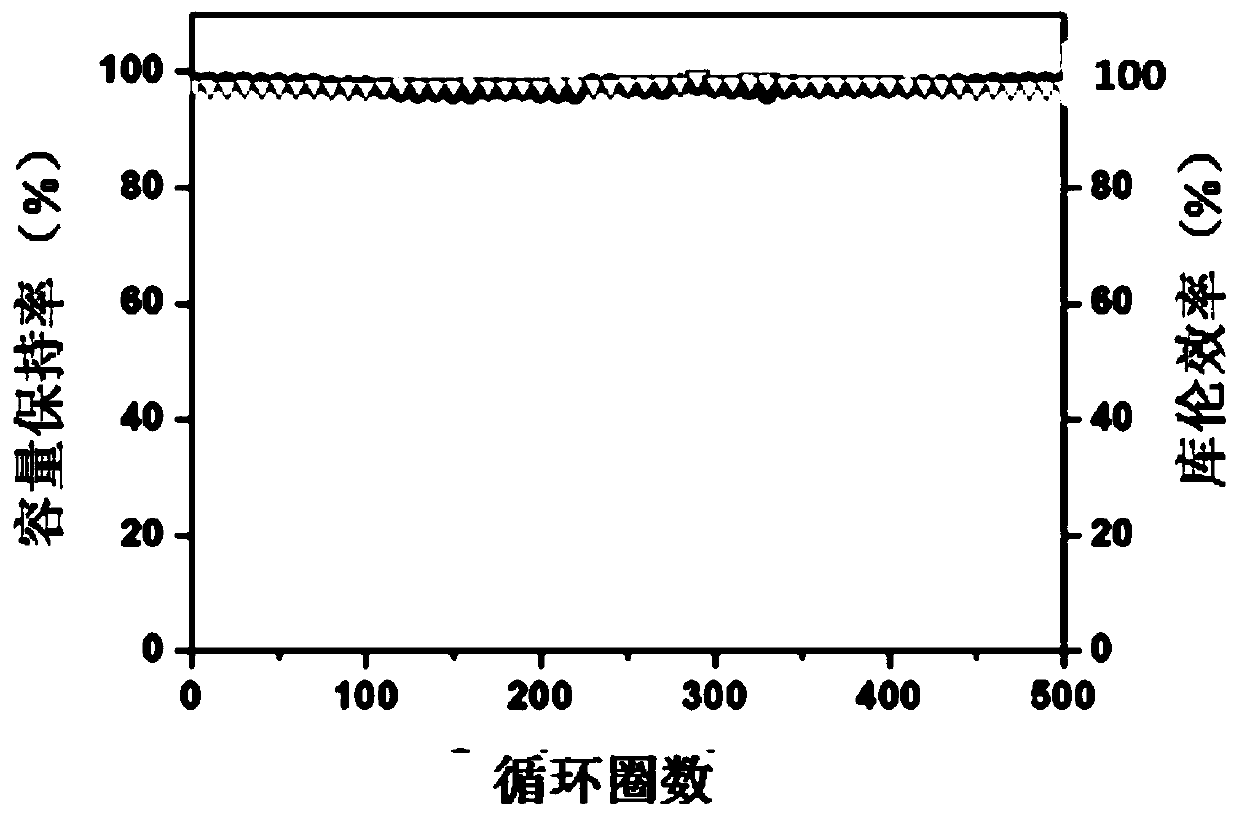
[0049] The above-mentioned aqueous metal ion battery was subjected to a constant rate charge and discharge test on a Land tester (Wuhan Xinnuo Electronics Co., Ltd.), the char...
Embodiment 3
[0052] Dimethyl sulfone, LiClO 4 (anhydrous) and H 2 O was mixed according to the molar ratio of 2:1:0.5, heated and stirred at 80°C, and allowed to cool to room temperature to obtain an aqueous electrolyte.
[0053] Then, with Mo 6 S 8 as the negative electrode, LiMn 2 o 4 As the positive electrode, a full battery is assembled without a separator. Wherein, the positive and negative electrode slurries are prepared according to m (active material): m (polyvinylidene fluoride, PVDF): m (acetylene black) = 75:10:15, and the prepared slurry is applied to the Ti grid , the mass of the positive electrode is about 5-8mg, and the mass of the negative electrode is 30%-40% greater than that of the positive electrode. The coated pole pieces were placed in an oven at 80° C. and baked in an air atmosphere for 13 hours to obtain a water-based metal ion battery.
[0054] The above-mentioned aqueous metal ion battery was subjected to a constant rate charge and discharge test on a Land ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| retention rate | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com