Aceglutamide injection with good stability and preparation method of aceglutamide injection
A technology for acetoglutamine and injection, applied in the field of acetoglutamine injection and preparation thereof, can solve the problems of decreased content, increased pH value, affecting the popularization and application of acetylglutamine injection, etc. Stable, stable drug quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
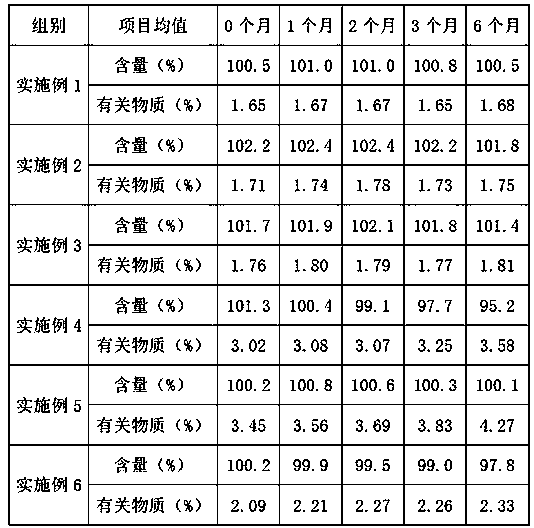
Embodiment 1
[0015] Embodiment 1: the formula of the acetylglutamine injection of every 10000ml is as follows:
[0016] Acetylglutamine 500g, sodium hydroxide 90g, lactose 4.6g, mannitol 7.5g, nicotinamide 10.5g, meglumine 4.2g, water for injection to 10000ml;
[0017] Its preparation method is as follows:
[0018] (1) Add sodium hydroxide and lactose to water for injection at 75°C respectively to form a 6.2 mol / L sodium hydroxide solution and a 3.4% lactose solution by mass percentage, then mix them and stir evenly to obtain a mixed solution a;
[0019] (2) Dissolve mannitol, nicotinamide, and meglumine with 500ml of 45°C water for injection, and let it stand for 3 hours to obtain mixed solution b;
[0020] (3) Dissolve acetylglutamine in 6500ml of water for injection with a water temperature of 75°C, then add mixed solution a and mixed solution b, then add water for injection to the full amount, and stir evenly at 75°C to obtain a medicinal solution;
[0021] (4) The liquid medicine is...
Embodiment 2
[0023] Embodiment 2: the formula of the acetylglutamine injection of every 10000ml is as follows:
[0024] Acetylglutamine 500g, sodium hydroxide 90g, lactose 1.2g, mannitol 4.5g, nicotinamide 7.5g, meglumine 2.4g, water for injection to 10000ml;
[0025] Its preparation method is as follows:
[0026] (1) Add sodium hydroxide and lactose to water for injection at 70°C respectively to form a 5.5 mol / L sodium hydroxide solution and a 2.1% lactose solution by mass percentage, and mix them evenly to obtain a mixed solution a;
[0027] (2) Dissolve mannitol, nicotinamide, and meglumine with 400ml of 40°C water for injection, and let it stand for 2 hours to obtain mixed solution b;
[0028] (3) Dissolve acetylglutamine in 6000ml of water for injection with a water temperature of 70°C, then add mixed solution a and mixed solution b, then add water for injection to the full amount, and stir evenly at 70°C to obtain a medicinal solution;
[0029] (4) The liquid medicine is first pass...
Embodiment 3
[0031] Embodiment 3: the formula of the acetylglutamine injection of every 10000ml is as follows:
[0032] Acetylglutamine 500g, sodium hydroxide 90g, lactose 5.9g, mannitol 10.5g, nicotinamide 12.3g, meglumine 5.6g, add water for injection to 10000ml;
[0033] Its preparation method is as follows:
[0034] (1) Add sodium hydroxide and lactose to water for injection at 80°C respectively to form a 7.2 mol / L sodium hydroxide solution and a 4.6% lactose solution by mass percentage, then mix them and stir evenly to obtain a mixed solution a;
[0035] (2) Dissolve mannitol, nicotinamide, and meglumine with 600ml of 50°C water for injection, and let it stand for 4 hours to obtain mixed solution b;
[0036] (3) Dissolve acetylglutamine in 7000ml of water for injection with a water temperature of 80°C, then add mixed solution a and mixed solution b, then add water for injection to the full amount, and stir evenly at 80°C to obtain a medicinal solution;
[0037] (4) The liquid medici...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com