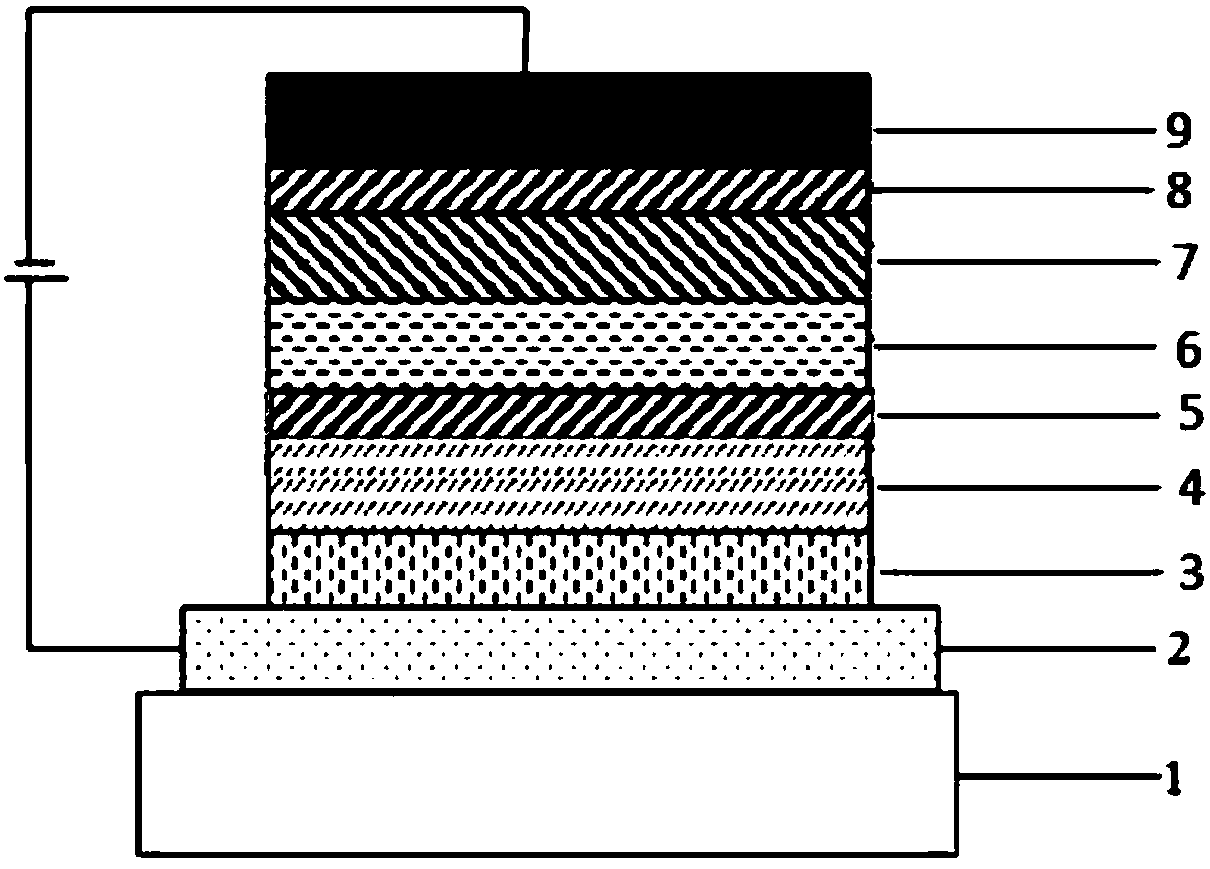
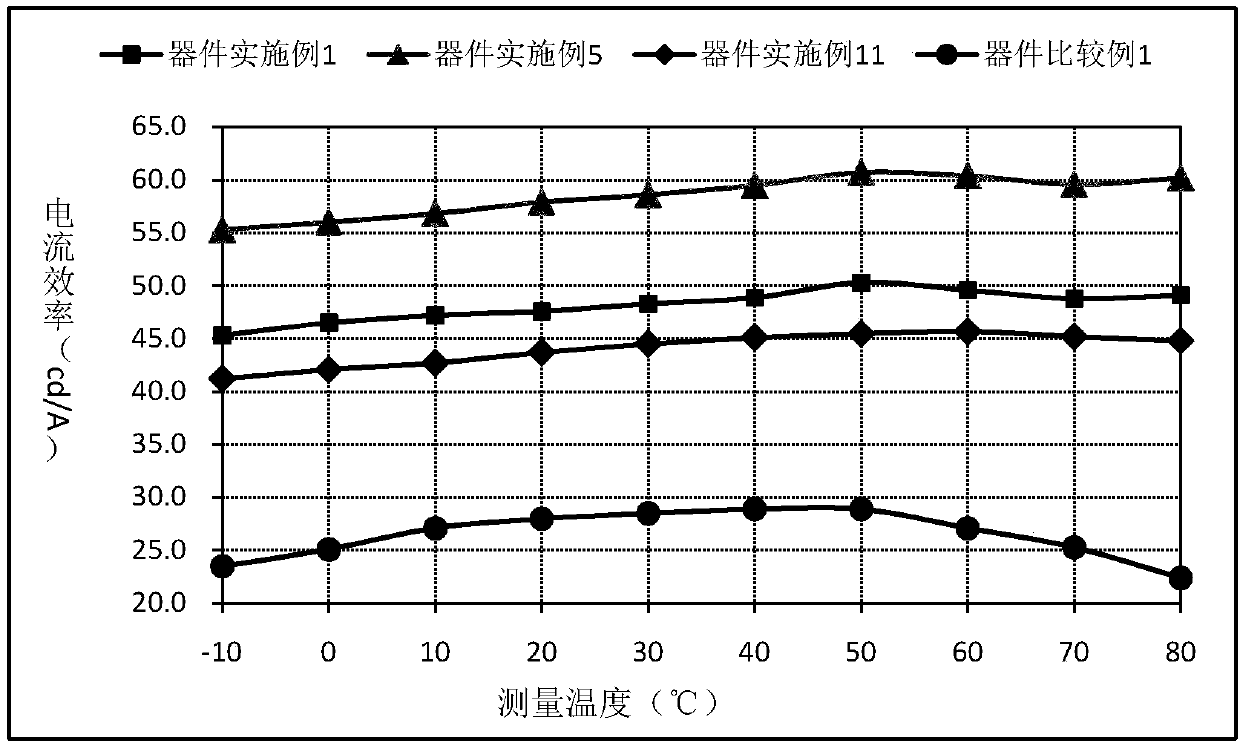
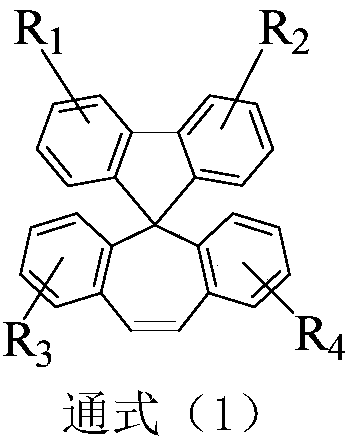
Organic compound containing spirofluorene dibenzocycloheptene and application of organic compound
A technology of benzocycloheptenene fluorene and organic compounds, which is applied in the preparation of organic compounds, organic chemistry, preparation of amino compounds, etc., and can solve different problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the synthesis of intermediate BI:
[0054]
[0055] Weigh raw material O and raw material P and dissolve them with toluene, then add Pd 2 (dba) 3 , P(Ph) 3 and sodium tert-butoxide; under an inert atmosphere, react the mixed solution of the above reactants at a reaction temperature of 90-110°C for 10-24 hours, cool and filter the reaction solution, spin the filtrate, and pass through a silica gel column to obtain the intermediate BI ; The molar ratio of the raw material O to the raw material P is 1:(1.0~1.5); Pd 2 (dba) 3 The molar ratio to raw material O is (0.006~0.02):1, and the molar ratio of sodium tert-butoxide to raw material P is (2.0~3.0):1; P(Ph) 3 The molar ratio with raw material O is (2.0~3.0):1;
[0056] Take the synthesis of intermediate B1 as an example:
[0057]
[0058] In a 250ml three-neck flask, under the protection of nitrogen, add 0.01mol raw material O1, 0.012mol raw material P1, 150ml toluene and stir to mix, then add 5...
Embodiment 2
[0059] Embodiment 2: the synthesis of intermediate BII:
[0060]
[0061] The concrete preparation method of above-mentioned reaction is: take intermediate BI and raw material N, dissolve with toluene; Add Pd 2 (dba) 3 , P(Ph) 3 , sodium tert-butoxide; under an inert atmosphere, react the mixed solution of the above-mentioned reactants at 95-110° C. for 10-24 hours, cool and filter the reaction solution, spin the filtrate, and pass through a silica gel column to obtain intermediate C; The molar ratio of the intermediate BI to the raw material N is 1:(1.0~1.5), Pd 2 (dba) 3 The molar ratio to the intermediate BI is (0.006~0.02):1, P(Ph) 3 The molar ratio of sodium tert-butoxide to intermediate BI is (0.006-0.02):1, and the molar ratio of sodium tert-butoxide to intermediate BI is (1.0-3.0):1.
[0062] Under nitrogen atmosphere, weigh intermediate C, bis(pinacolate) diboron, Pd(dppf)Cl 2 1. Potassium acetate was dissolved in toluene, reacted at 100-120°C for 12-24 hours...
Embodiment 3
[0070] Embodiment 3: the synthesis of intermediate AI:
[0071]
[0072] In an oven-dried flask, 0.01 mol of raw material N was dissolved in 500 mL of anhydrous THF, and the reaction mixture was cooled to -78°C, then, 34.3 mL of n-BuLi (2.5 mol / L, hexane solution), stirred for 1 h, then 0.01 mol of starting material M was dissolved in THF and added dropwise at -70°C. After the addition was complete, the reaction mixture was gradually warmed to room temperature, extracted with ammonium chloride and concentrated on a rotary evaporator, 500 mL of acetic acid was carefully added to the concentrated solution, followed by 100 mL of fuming hydrochloric acid. The mixture was heated to 75 °C for 5 h during which a white solid came out, then the mixture was cooled to room temperature and the precipitated solid was filtered off with suction, washed with methanol and the residue was dried at 40 °C under reduced pressure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com