Reactive liquid crystal monomer compound, preparation method and application
A reactive liquid crystal and compound technology, applied in the field of liquid crystal, can solve the problems of complex structure, unfavorable industrialization and high cost, and achieve the effect of simple synthesis route and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
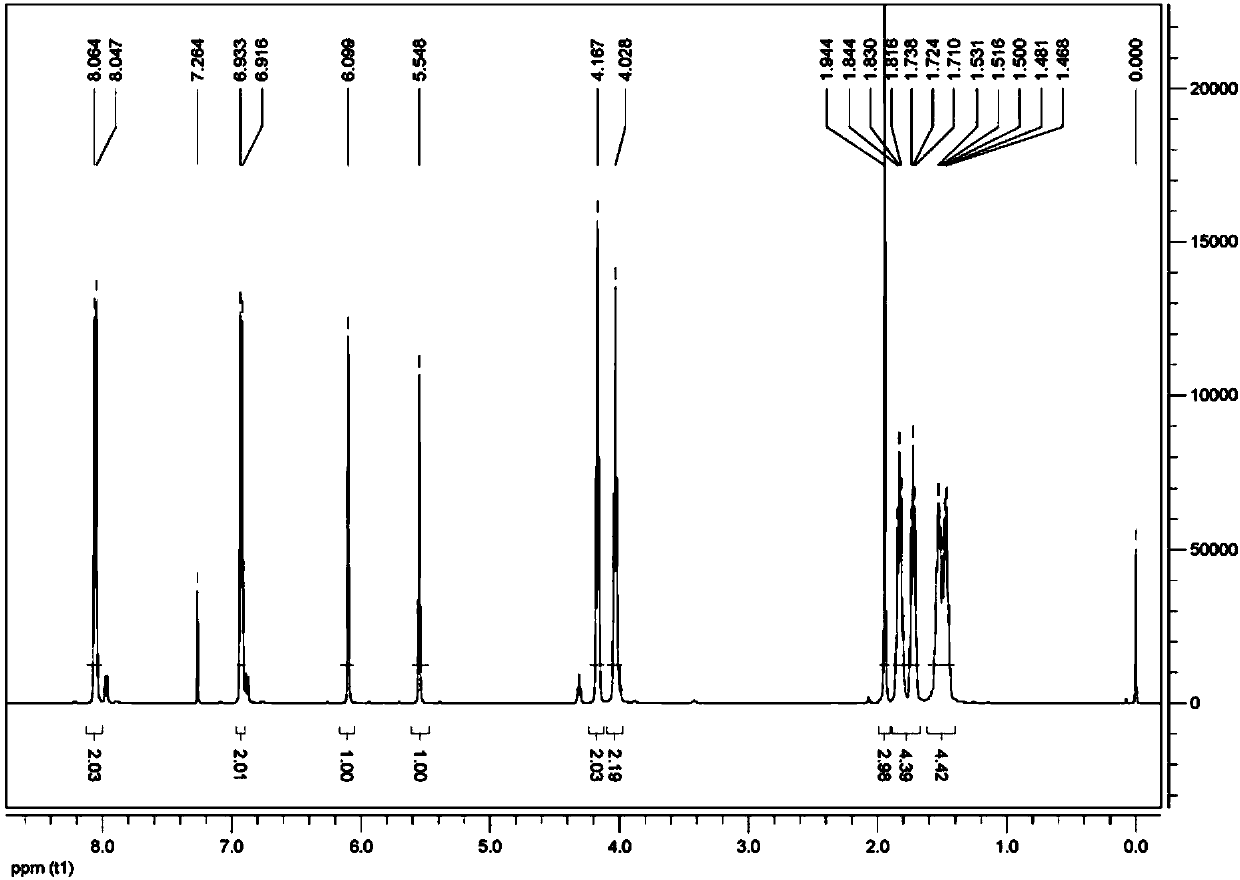
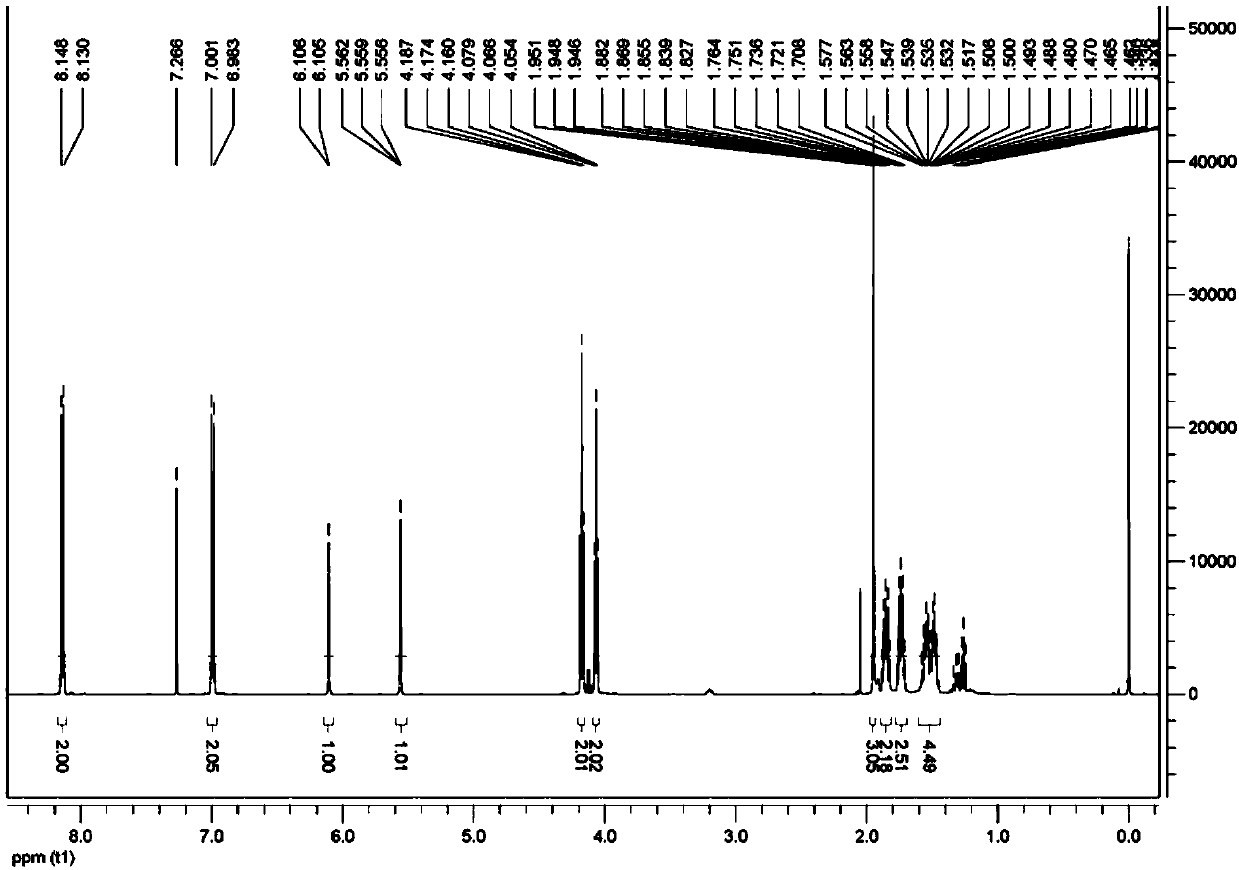
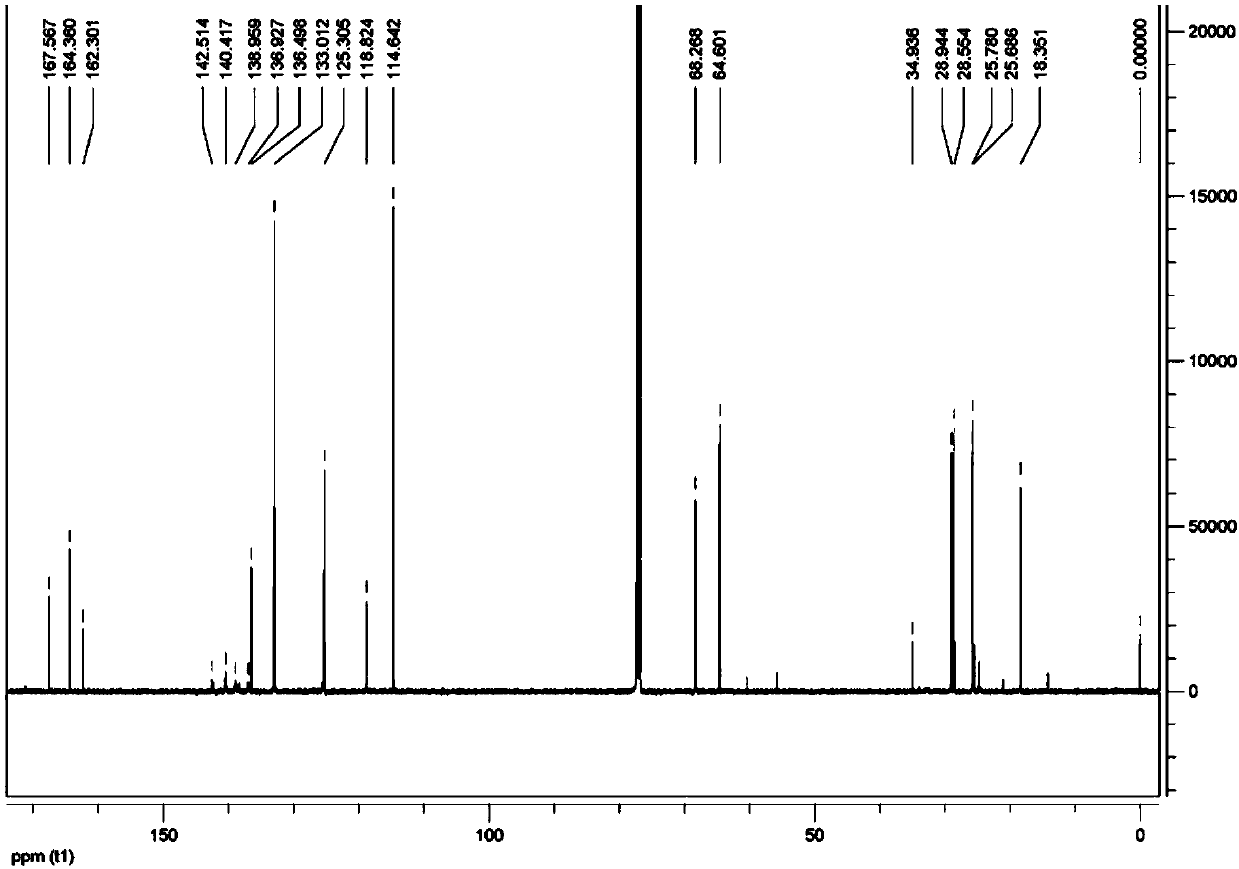
[0046] This embodiment provides a reactive liquid crystal monomer compound, the structural formula of which is shown in the abbreviation code M6BAF, that is, the compound 4-(6-(methacryloyloxy)hexyloxy)pentafluorophenylbenzoate .
[0047] The preparation method of the compound of the present embodiment specifically comprises the following steps:
[0048] Step 1: Synthesis of 4-(6-acetoxyhexyloxy)-benzoic acid methyl ester
[0049] Add 110g of N,N-dimethylformamide (DMF for short) into a 250mL three-necked flask, start stirring, and add 12.6g of 6-chlorohexanol acetate, 9g of methyl p-hydroxybenzoate, 0.5g of potassium iodide, carbonic acid Potassium 13.50g. Turn on heating to raise the temperature to 130°C, control the temperature at 127-133°C and react for 2 hours, take samples every half hour for gas chromatography detection, and stop the reaction when the raw material methyl p-hydroxybenzoate is less than 0.5%. When the temperature was lowered to 40°C, filter while it wa...
Embodiment 2
[0067] This embodiment provides a reactive liquid crystal monomer compound, the structural formula of which is shown in the abbreviation code M2BAF, that is, the compound 4-(2-(methacryloyloxy)ethoxy)pentafluorophenylbenzoate .
[0068] The preparation method of the compound of the present embodiment specifically comprises the following steps:
[0069] Step 1: Synthesis of 4-(2-acetoxyethoxy)-benzoic acid methyl ester
[0070] Add 110 g of DMF to a 250 mL three-necked flask, start stirring, and add 15 g of 2-chloroethanol acetate, 16.76 g of methyl p-hydroxybenzoate, 0.5 g of potassium iodide, and 16.50 g of potassium carbonate. Turn on heating and raise the temperature to 130°C, control the temperature at 127-133°C and react for 2 hours, take a sample every half hour for gas chromatography detection, and stop the reaction when the raw material methyl p-hydroxybenzoate is less than 0.5%. When the temperature was lowered to 40°C, filter while it was hot to remove inorganic sa...
Embodiment 3
[0081] This embodiment provides a reactive liquid crystal monomer compound, the structural formula of which is shown as the abbreviation code A3BAF, that is, the compound 4-(6-(acryloyloxy)propoxy)pentafluorophenylbenzoate.
[0082] The preparation method of the compound of the present embodiment specifically comprises the following steps:
[0083] Step 1: Synthesis of 4-(3-acetoxypropoxy)-benzoic acid methyl ester
[0084] Add 110 g of DMF to a 250 mL three-necked flask, start stirring, and add 15 g of 3-chloropropanol acetate, 15.88 g of methyl p-hydroxybenzoate, 0.5 g of potassium iodide, and 15.20 g of potassium carbonate. Turn on heating and raise the temperature to 130°C, control the temperature at 127-133°C and react for 2 hours, take a sample every half hour for gas chromatography detection, and stop the reaction when the raw material methyl p-hydroxybenzoate is less than 0.5%. When the temperature is lowered to 40°C, filter while it is hot to remove inorganic salts, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com