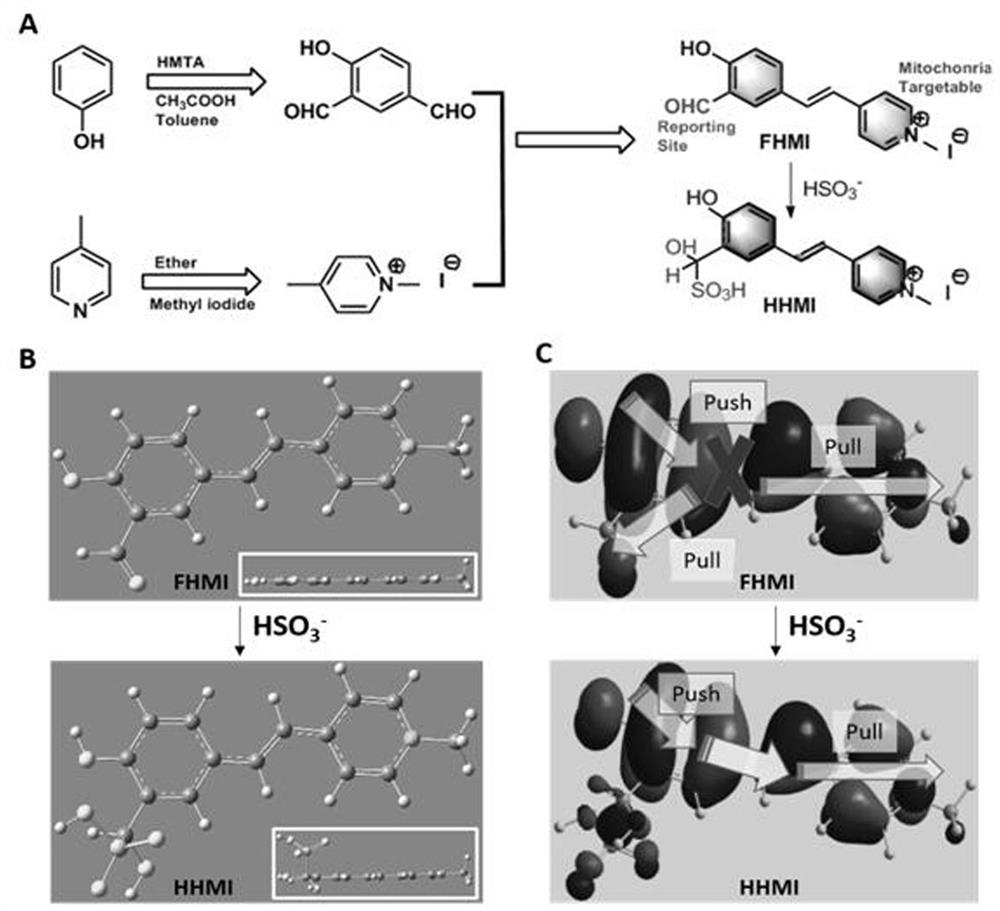
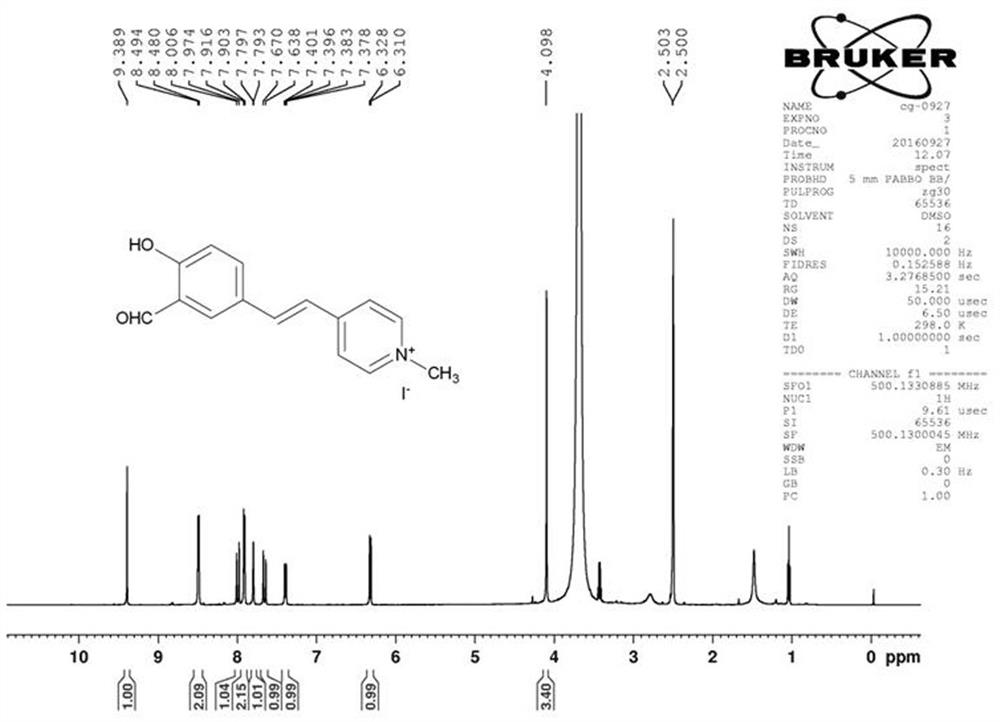
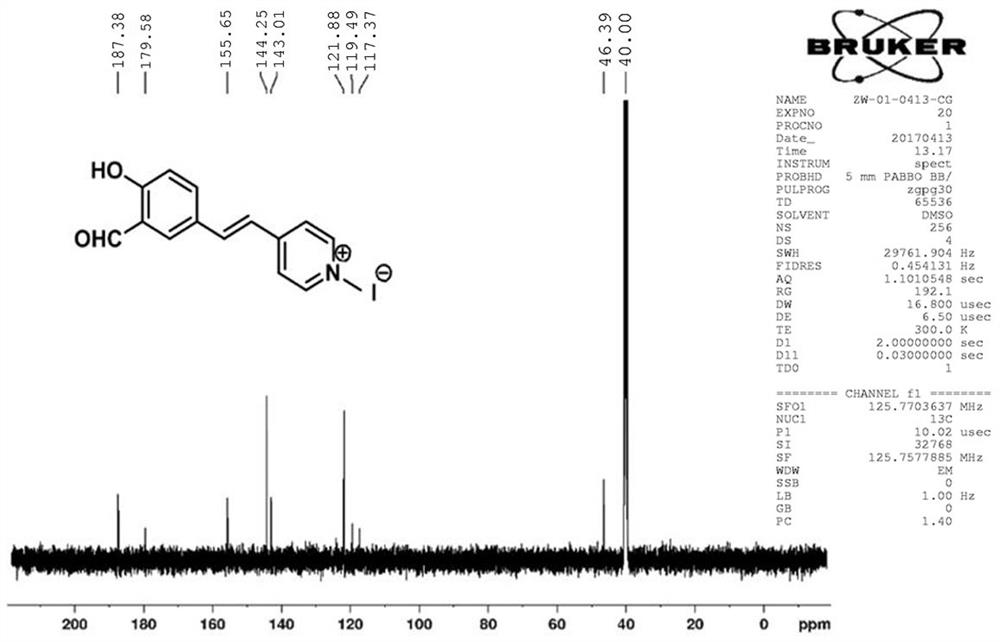
A "triangular electronic push-pull" mitochondria-targeted assay for so 2 Fluorescent probe and its preparation method and application
A technology of fluorescent probes and mitochondria, which is applied in the field of analytical chemistry, can solve the problems of electron-donating groups detaching from the conjugation plane, affecting fluorescence intensity and sensitivity, etc., and achieves the effect of being suitable for optical detection, novel and unique luminescent mechanism, and novel principle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of FHMI molecular probe
[0060] A "triangular electronic push-pull" mitochondria-targeted detection of SO 2 A method for preparing a fluorescent probe, the steps comprising:
[0061] (1) Dissolve 300mg of phenol in a mixed solution of 20ml of toluene and 25ml of glacial acetic acid, then add 983mg of hexamethylenetetramine, mix well, heat and reflux for 18h, until the color of the solution turns orange-red. After cooling to room temperature, 6M hydrochloric acid was added, followed by extraction with ethyl acetate (70 ml, extracted three times). The extracted organic layer was washed with 150ml of saturated brine, then dried with anhydrous sodium sulfate, and after the solvent was removed by rotary evaporation, it was purified through a silica gel column (dissolved in petroleum ether, and the eluent was petroleum ether: ethyl acetate = 10:1), to obtain a yellow solid, namely 4-hydroxyisophthalaldehyde.
[0062] (2) Add 4ml of 4-picoline, 12...
Embodiment 2
[0068] Embodiment 2: Preparation of FHMI molecular probe
[0069] A "triangular electronic push-pull" mitochondria-targeted detection of SO 2 A method for preparing a fluorescent probe, the steps comprising:
[0070] (1) Dissolve 240mg of phenol in a mixed solution of 20ml of toluene and 20ml of glacial acetic acid, then add 720mg of hexamethylenetetramine, mix well, heat and reflux for 16h, until the color of the solution turns orange-red. After cooling to room temperature, 7M hydrochloric acid was added, followed by extraction with ethyl acetate (40 ml, extracted three times). The extracted organic layer was washed with 120ml of saturated brine, then dried with anhydrous sodium sulfate, and after the solvent was removed by rotary evaporation, it was purified through a silica gel column (dissolved in petroleum ether, and the eluent was petroleum ether: ethyl acetate = 10:1), to obtain a yellow solid, namely 4-hydroxyisophthalaldehyde.
[0071] (2) Add 4ml of 4-picoline, 16...
Embodiment 3
[0075] Embodiment 3: Preparation of FHMI molecular probe
[0076] A "triangular electronic push-pull" mitochondria-targeted detection of SO 2 A method for preparing a fluorescent probe, the steps comprising:
[0077] 1) Dissolve 350mg of phenol in a mixed solution of 20ml of toluene and 30ml of glacial acetic acid, then add 1.400g of hexamethylenetetramine, mix well, heat and reflux for 20h, until the color of the solution turns orange-red. After cooling to room temperature, 8M hydrochloric acid was added, followed by extraction with ethyl acetate (100ml, three extractions). The extracted organic layer was washed with 200ml of saturated brine, then dried with anhydrous sodium sulfate, and after the solvent was removed by rotary evaporation, it was purified through a silica gel column (dissolved in petroleum ether, and the eluent was petroleum ether: ethyl acetate = 10:1), to obtain a yellow solid, namely 4-hydroxyisophthalaldehyde.
[0078] (2) Add 4ml of 4-picoline, 14ml o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com