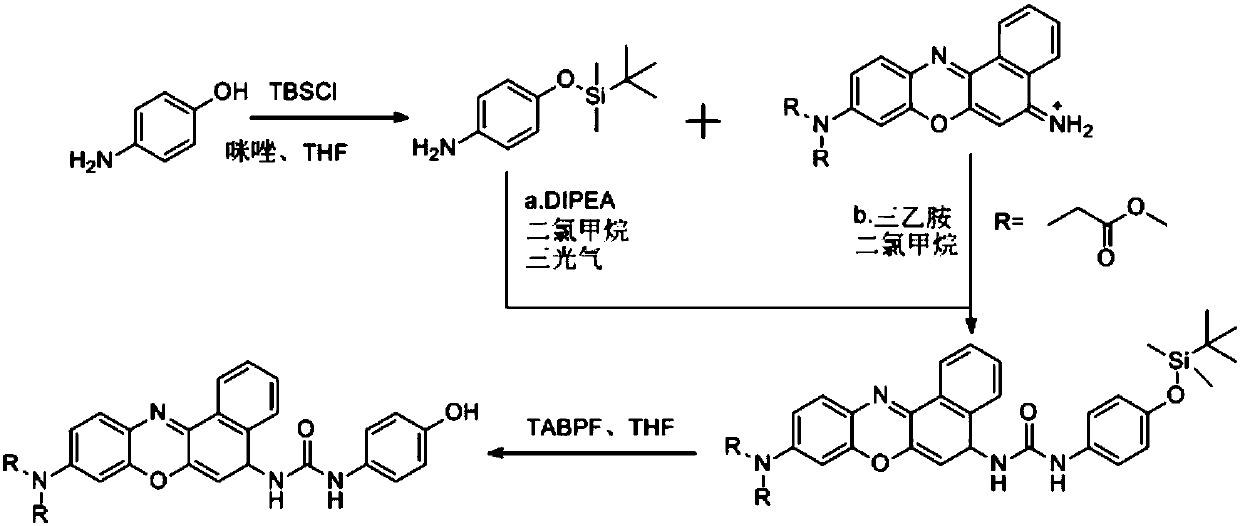
Fluorescent probe and preparation method thereof, and applications of fluorescent probe in detection of tyrosinase
A fluorescent probe and reaction technology, applied in the field of analysis and detection, can solve the problems that fluorescent probes are easily interfered by external factors, are not suitable for accurate detection of tyrosinase, and have poor detection sensitivity of fluorescent probes, and achieve an intuitive detection mode. , Improve the detection accuracy and accuracy, and facilitate the effect of popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
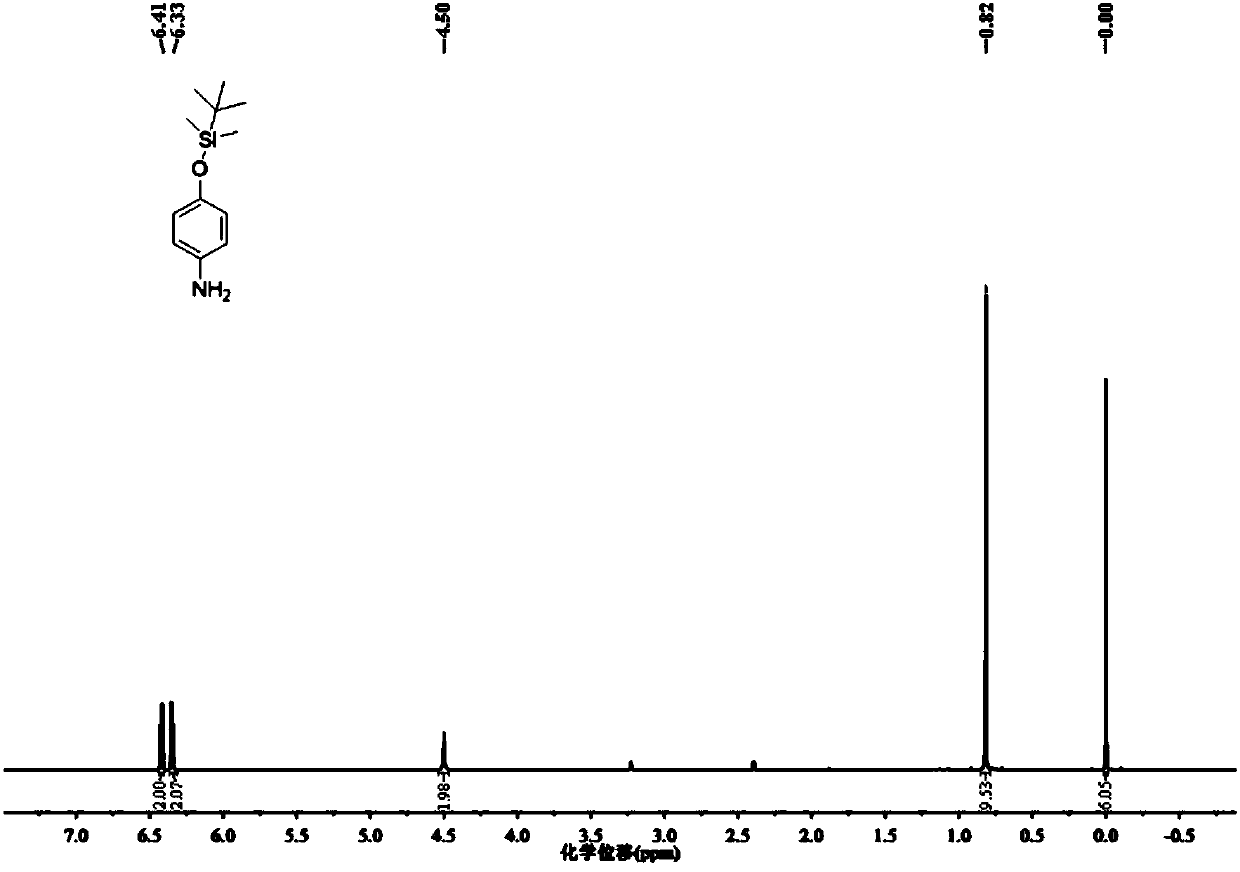
[0047] (1) Dissolve 1.0g of 4-aminophenol and 0.936g of imidazole in tetrahydrofuran, add 1.797g of tert-butyldimethylchlorosilane, stir rapidly to form a white precipitate, continue to react for 30 minutes, remove the solvent by rotary evaporation, wash with water, Then extract with ethyl acetate, the gained organic layer is washed with water and saturated sodium chloride solution, the organic phase is dried again, and the organic solvent is removed by rotary evaporation, and the obtained solid is purified by silica gel column chromatography (petroleum ether: ethyl acetate, V / V =10:1), obtain colorless viscous product 4-(tert-butyldimethylsilaoxy)aniline 1.842g (yield rate is 90.0%); This product is characterized by proton nuclear magnetic resonance spectrum, nuclear magnetic resonance The resonance spectrum is as figure 2 shown;
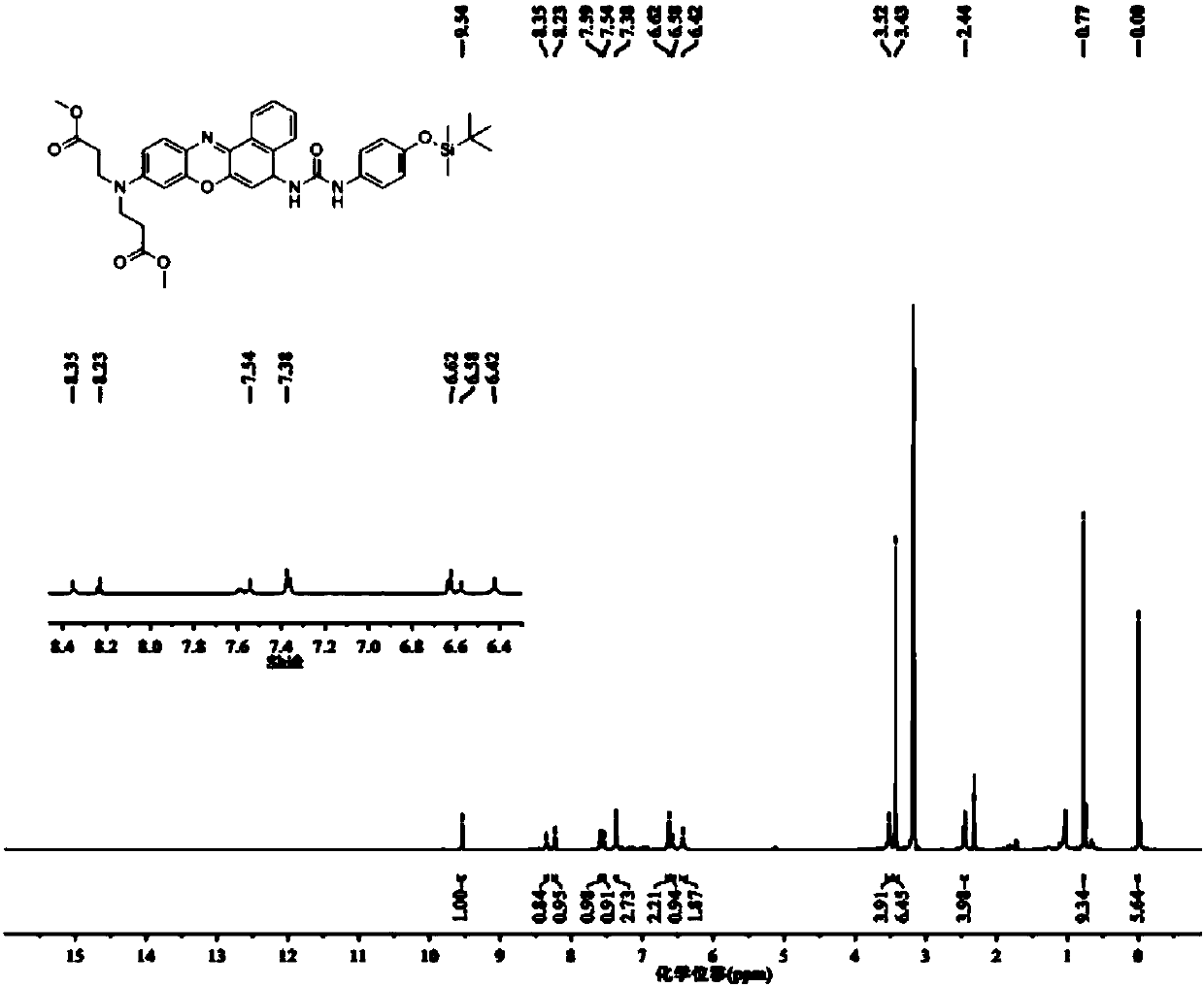
[0048] (2) Dissolve 0.210g of 4-(tert-butyldimethylsilyloxy)aniline and 1.117g of triphosgene in 15ml of dichloromethane, and add 959μl of N,N-d...
Embodiment 2
[0055] (1) Dissolve 1.0g of 4-aminophenol and 0.967g of imidazole in tetrahydrofuran, add 1.866g of tert-butyldimethylchlorosilane, stir rapidly to form a white precipitate, continue the reaction for 45 minutes, and remove the solvent by rotary evaporation to obtain a solid Purified by silica gel column chromatography (petroleum ether: ethyl acetate, V / V=10:1), to obtain a colorless viscous product 4-(tert-butyldimethylsilyloxy)aniline 1.887g (yield was 92.2%);
[0056] (2) Dissolve 0.210g of 4-(tert-butyldimethylsilyloxy)aniline and 1.117g of triphosgene in 15ml of dichloromethane, and add 959μl of N,N-diiso The organic solution of propylethylamine was first reacted at low temperature for 1 hour, then reacted at room temperature for 2.5 hours, and spin-dried under reduced pressure to obtain a reaction intermediate product;
[0057] (3) Under the protection of inert gas, dissolve 0.470g 9-[bis(3-methoxy-3-propionyl)amino]-5H-benzo[α]phenoxazine-5-imine salt in Add 180 μl of tr...
Embodiment 3
[0061] (1) Dissolve 1.0g of 4-aminophenol and 0.998g of imidazole in tetrahydrofuran, add 1.932g of tert-butyldimethylsilyl chloride, stir rapidly to form a white precipitate, continue the reaction for 1 hour, and remove the solvent by rotary evaporation to obtain a solid Purified by silica gel column chromatography (petroleum ether: ethyl acetate, V / V=10:1), to obtain a colorless viscous product 4-(tert-butyldimethylsilyloxy)aniline 1.862g (yield is 91.1%);
[0062] (2) Dissolve 0.210g of 4-(tert-butyldimethylsilyloxy)aniline and 1.117g of triphosgene in 20ml of dichloromethane, and add 959μl of N,N-diiso The organic solution of propylethylamine was first reacted at low temperature for 1 hour, then reacted at room temperature for 3 hours, and spin-dried under reduced pressure to obtain a reaction intermediate product;
[0063] (3) Under the protection of inert gas, dissolve 0.490g 9-[bis(3-methoxy-3-propionyl)amino]-5H-benzo[α]phenoxazine-5-imine salt in Add 188 μl of triet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com