Amphipathic tri-block copolymer having pH responsiveness and preparation method of same
An amphiphilic and responsive technology, applied in the direction of non-active ingredient medical preparations, pharmaceutical formulations, emulsion delivery, etc., can solve the problems that hinder the development and application of oral drugs, are easily degraded by acids and enzymes, and have low bioavailability and other problems, to achieve the effect of less catalyst consumption, good stability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
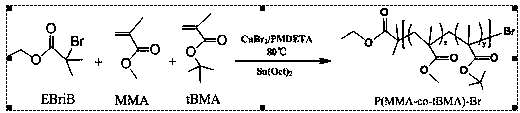
[0067] (1) Synthesis of macroinitiator poly(methyl methacrylate-co-tert-butyl methacrylate)-bromine (P(MMA-co-tBMA)-Br)
[0068] Weigh CuBr 2 (9.0 mg, 0.04mmol) was placed in a 50ml dry eggplant-shaped bottle, sealed with an inversion rubber stopper, and after pumping air-argon for 3 times, the solvent toluene (10 mL), single MMA (3.708 mL, 35 mmol), tBMA (4.765 mL, 30 mmol) and catalyst ligand pentamethyldiethylenetriamine (PMDETA) (0.105 mL, 0.5 mmol) were added to the bottle, stirred for 25 min, and a complex was formed. compound catalyst, the reducing agent Sn(Oct) 2 (0.195 mL, 0.6 mmol) mixed with 3 mL of toluene was injected into the reaction flask, and then stirred for 10 min. After 3 cycles of liquid nitrogen freezing and freezing-pumping-heating and thawing, argon gas was introduced, and the initiator was added dropwise with a syringe. Ethyl 2-bromoisobutyrate (EBriB) (0.147 mL, 1 mmol) was transferred to an oil bath at 80°C for 2 h. After the reaction was completed...
Embodiment 2
[0074] Gel permeation chromatography (GPC, Agilent 1260) to measure the number average molecular weight (Mn) and molecular weight distribution (Mw / Mn) of polymers
[0075] (1) Instrument and operation: guard column (PLgel 5 um), LC quantitative pump, separation column PLgel 5um MIXED-D (serialNo.0006160533-100), separation column PLgel 5um MIXED-D (serial No.0006160533-102), And RI differential refractive index detector. The column was calibrated with monodisperse polystyrene standard sample, the mobile phase (chromatographic grade tetrahydrofuran (THF), flow rate: 1.0mL / min), and the column temperature was 30 o c.
[0076] (2) Preparation of sample solution: Accurately weigh 5mg of sample and dissolve it in 1ml of chromatographic grade tetrahydrofuran (THF) to prepare a sample solution with a concentration of 5m / ml.
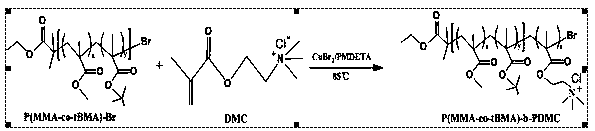
[0077] (3) Measurement of Example 1 (poly(methyl methacrylate-co-tert-butyl methacrylate)-bromine (P(MMA-co-tBMA)-Br), poly(methyl methacrylate-co - tert-but...
Embodiment 3
[0079] Determination of Critical Micelle Concentration of pH Responsive Amphiphilic Block Copolymers by Pyrene Fluorescent Probe Method
[0080] (1) Preparation of 3×10-5M (M=mol / l) pyrene solution: Weigh 2.43mg of pyrene and dissolve it in 10ml of acetone solution, pipette it into a 100ml volumetric flask, dilute with acetone solution to make a concentration of 12 ×10 -5 The pyrene solution of M is ready for use, take 5ml 12×10 -5 M pyrene solution, diluted with acetone to 3×10 -5 M.
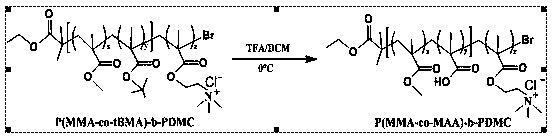
[0081] (2) Preparation of sample solution: Weigh 10 mg of amphiphilic block copolymer poly(methyl methacrylate-co-tert-butyl methacrylate)-polymethacryloxyethyltrimethylammonium chloride Dissolve P(MMA-co-MAA)-b-PDMC in 3ml of acetone, add 10ml of deionized aqueous solution with pH 6.8 under stirring, and place it at room temperature for 24 hours to completely volatilize the acetone to obtain a 1mg / ml polymer mother solution , and then dilute the polymer mother liquor into a series of 0.000...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| critical micelle concentration (mass) | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com