Cyanide-free alkaline copper electroplating composition and preparation method thereof
A composition and alkali copper technology, applied in the field of electroplating, can solve the problems of poor stability, brittle coating, easy to mold, etc., and achieve the effect of good stability of the plating solution, bright and flat coating, and good bonding force of the coating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] As a preferred technical solution of the present invention, the preparation method of (1,2-benzisothiazolin-3-one)-propyne at least comprises the following steps:
[0065] a. Take o-aminothiophenol and urea, raise the temperature to 155-165° C., stop the reaction after stirring for 1 hour, and recrystallize the obtained product twice with hot water to obtain 1,2-benzisothiazolin-3-one. The molar ratio of the above-mentioned o-aminothiophenol and urea is 1:1.2;
[0066] b. Dissolve 1,2-benzisothiazolin-3-one in acetone, slowly add potassium carbonate, stir for 15 to 30 minutes, add 3-chloropropyne, heat and reflux for 4 hours, the 1,2- The molar ratio of benzisothiazolin-3-one, potassium carbonate, 3-chloropropyne and acetone is 1:1:1:3; then the mixture is cooled to 0°C, and 100 mL of ice-water mixture is poured into it, Control the temperature at 0-10°C and stir for 1 h; collect the formed precipitate, filter it with deionized water, and wash until the pH is neutral t...
Embodiment 1
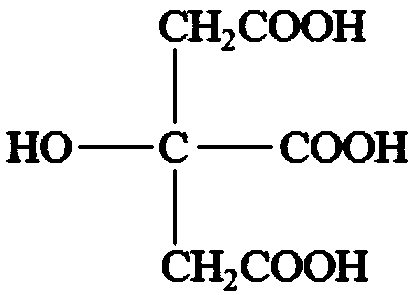
[0091] Example 1 provides a cyanide-free alkali copper electroplating composition. The raw materials for the preparation of 1 L of cyanide-free alkali copper electroplating composition include: divalent copper ion: 15g; citric acid: 185g; potassium sodium tartrate: 50g; potassium nitrate: 5g; 1,2-cyclohexanediaminetetraacetic acid: 0.5g; wetting agent: 0.2g; brightener: 1.2g; leveling agent: 0.1g; add deionized water to 1L.
[0092] Described divalent copper ion is provided by basic copper carbonate;
[0093] Described wetting agent is polyethylene glycol 1000;
[0094] The brightener is 1,4-butynediol;
[0095] The leveling agent is (1,2-benzisothiazolin-3-one)-propyne.
[0096] The preparation method of the (1,2-benzisothiazolin-3-one)-propyne at least comprises the following steps:
[0097] a. Take o-aminothiophenol and urea, raise the temperature to 160° C., stop the reaction after stirring for 1 hour, and recrystallize the obtained product twice with hot water to obtai...
Embodiment 2
[0104] Example 2 provides a cyanide-free alkali copper electroplating composition. The raw materials for the preparation of 1 L of cyanide-free alkali copper electroplating composition include: divalent copper ion: 17g; citric acid: 195g; potassium sodium tartrate: 60g; potassium nitrate: 6.5g; 1,2-cyclohexanediaminetetraacetic acid: 1g; wetting agent: 0.3g; brightener: 1.6g; leveling agent: 0.15g; add deionized water to 1L.
[0105] The divalent copper ions are provided by copper citrate;
[0106] Described wetting agent is myristyl betaine;
[0107] Described brightener is thiourea;
[0108] The leveling agent is (1,2-benzisothiazolin-3-one)-propyne.
[0109] The preparation method of the (1,2-benzisothiazolin-3-one)-propyne is the same as in Example 1.
[0110] The preparation method of the cyanide-free alkali copper electroplating composition is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Roughness | aaaaa | aaaaa |
| Surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com