Synthesis method of 2-chloro-5-chloromethyl pyridine
A technology for the synthesis of chloromethylpyridine and a synthesis method, which is applied in the field of synthesis of 2-chloro-5-chloromethylpyridine, can solve the problems of high production cost, many wastes, and long reaction routes, and achieve less wastes and low comprehensive cost , improve the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
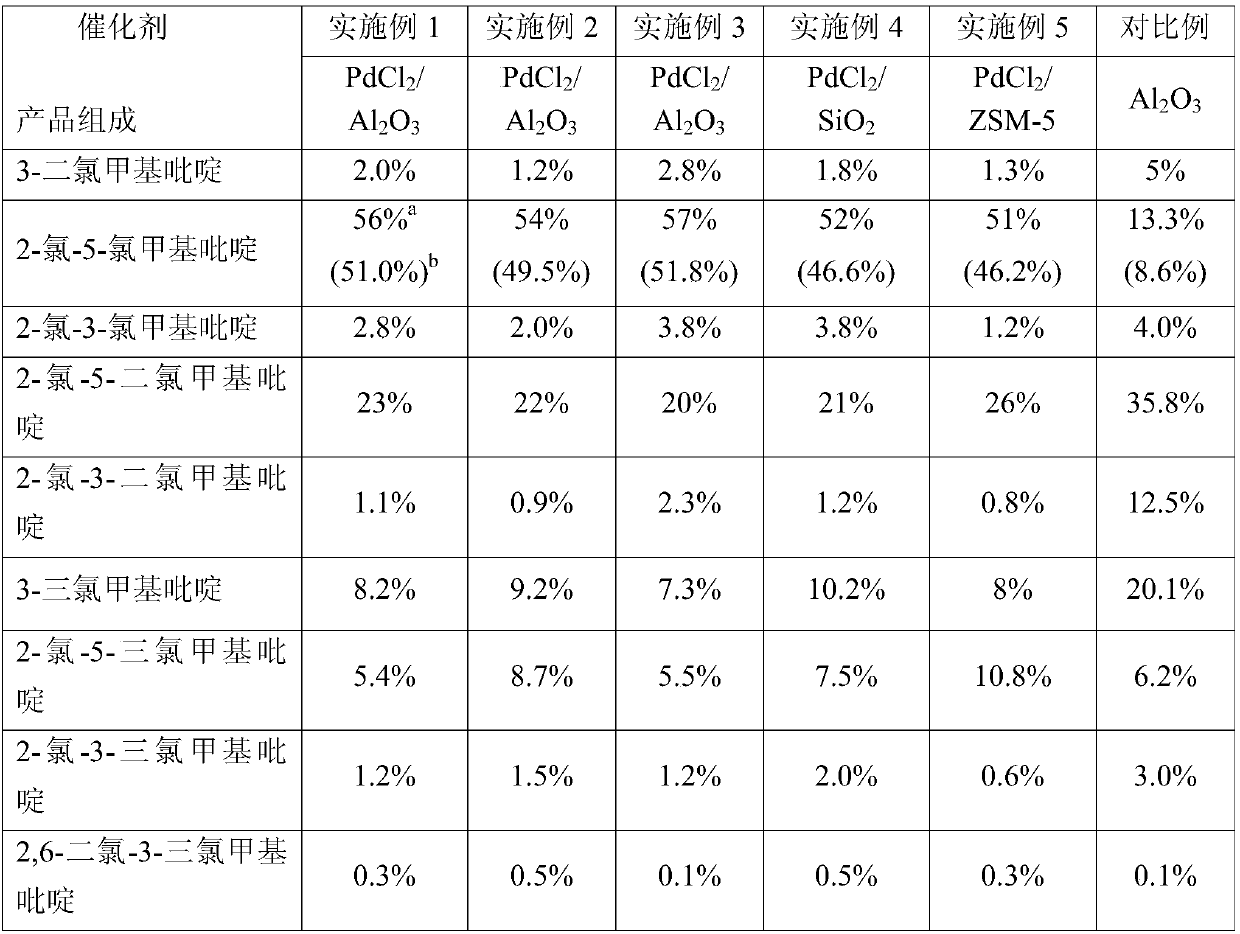
Embodiment 1
[0024] PdCl 2 / Al 2 o 3 (0.83%PdCl 2 ) catalyst preparation:
[0025] 100g alumina carrier (spherical, diameter 2mm, BET: 200m 2 / g), added to 150gPd 2+ Immerse in 0.333% palladium chloride aqueous solution at room temperature for 24 hours, dry at 110°C, and roast at 250°C for 5 hours to obtain PdCl 2 0.83% PdCl loading 2 / Al 2 o 3 catalyst.
[0026] Chlorination reaction:
[0027] Get 20g of the PdCl prepared above 2 / Al 2 o 3 The catalyst was added to a quartz tube reactor with a length of 20 cm and a diameter of 2.4 cm. The height of the catalyst bed was 8 cm. Turn on the resistance wire and heat until the temperature of the catalyst layer was stable at 250 ° C. -Methylpyridine (flow rate 10g / h, temperature 200°C), mixed with chlorine gas (flow rate 200mL / min), enters the catalyst layer for chlorination reaction, the residence time is 5s, and the reaction temperature automatically rises to 280°C±2°C After maintaining stability, the reaction system reached a st...
Embodiment 2
[0029] The operation method and process conditions are the same as in Example 1, except that the flow rate of the chlorine gas introduced is changed to 350mL / min, and the yield of the product 2-chloro-5-chloromethylpyridine is 49.5%.
Embodiment 3
[0031] The operation method and process conditions are the same as in Example 1, except that the nitrogen flow rate is changed to 400mL / min, and the yield of the product 2-chloro-5-chloromethylpyridine is 51.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com