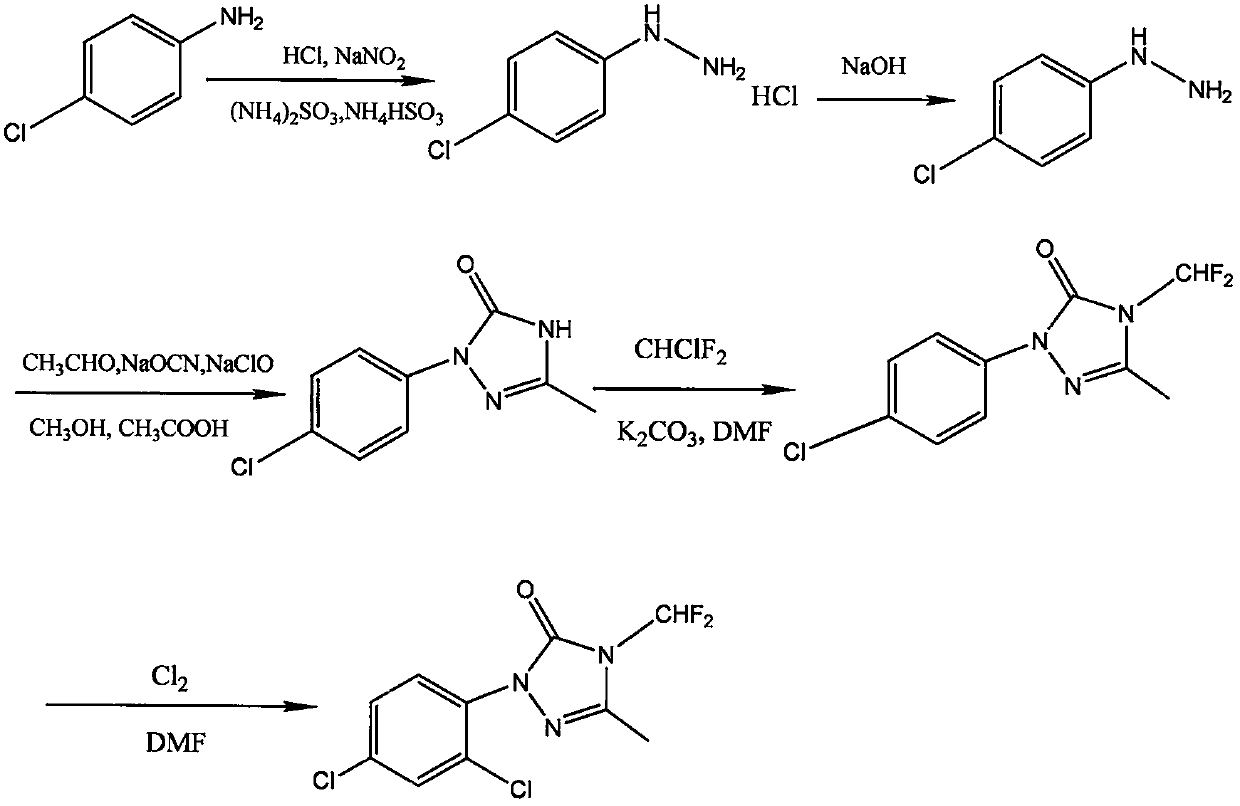
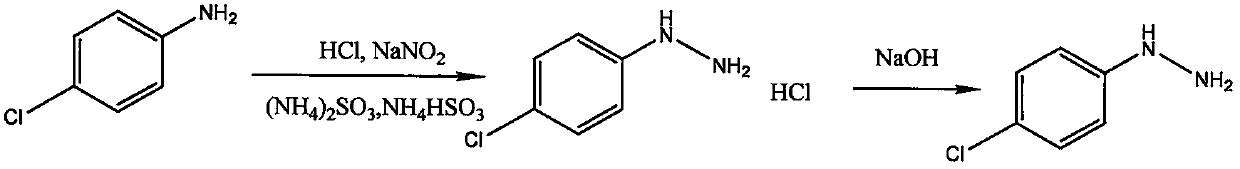
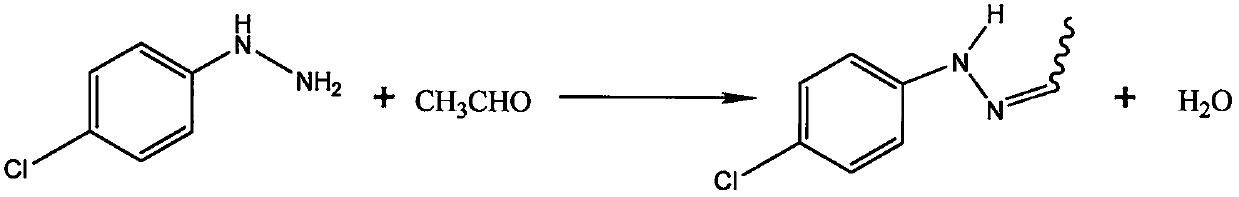
Method for synthesizing 1-(2,4-dichlorophenyl)-3-methyl-4-difluoromethyl-1,2,4-triazole-5-ketone
A technology of difluoromethyl and dichlorophenyl is applied in the field of synthesizing 1--3-methyl-4-difluoromethyl-1, which can solve the problem that trimethyl acetate and potassium cyanate are not easy to obtain in price, intermediate The product is prone to problems such as steric hindrance and low boiling point of dichloromethane, and achieves the effects of small steric hindrance, lower production costs, and fewer side reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] The following will clearly and completely describe the technical solutions in the embodiments of the present invention with reference to the accompanying drawings in the embodiments of the present invention. Obviously, the described embodiments are only some, not all, embodiments of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without making creative efforts belong to the protection scope of the present invention.
[0054] see figure 1 , a method for synthesizing 1-(2,4-dichlorophenyl)-3-methyl-4-difluoromethyl-1,2,4-triazol-5-one, using p-chloroaniline as raw material , synthesize p-chlorophenylhydrazine hydrochloride through diazotization, reduction, and salification, and obtain p-chlorophenylhydrazine through alkali neutralization. Under the condition of tert-butanol as solvent, p-chlorophenylhydrazine is mixed with acetaldehyde and sodium cyanate , Sodium hypochlorit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com