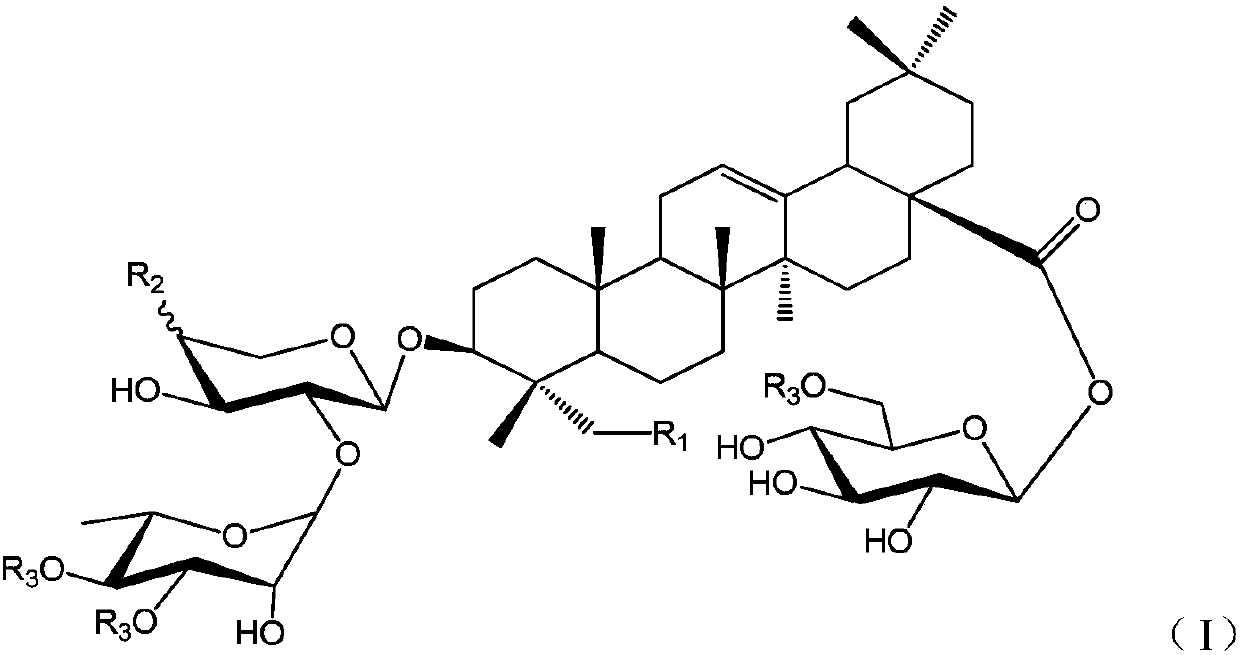
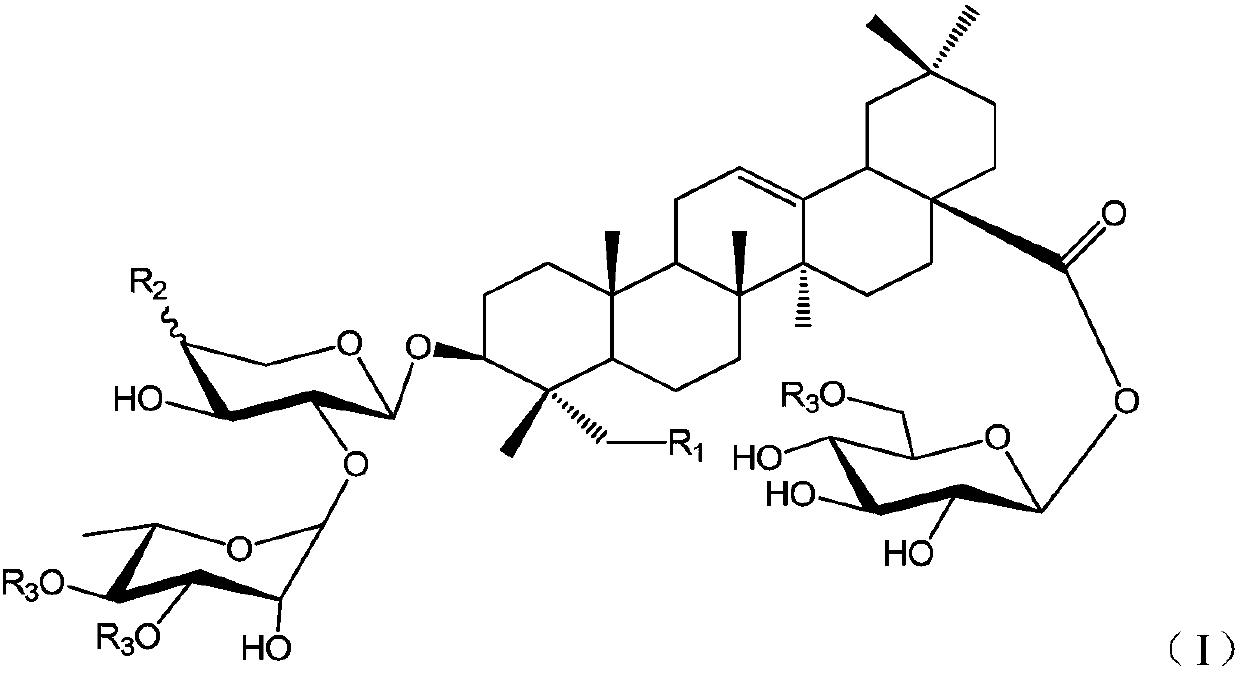
Oleanolic acid-type saponin compound and composition thereof
A technology of oleanolic acid and saponins, which is applied in the field of oleanolic acid type saponins and compositions thereof, can solve the problems of low oral bioavailability, large irritating side effects of the gastrointestinal tract, etc. Side effects, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Preparation of oleanolic acid saponins S1-S4
[0027] Prepared with reference to the method reported in the literature of the present invention: Liming Wang, et al. Synthesis and cytotoxicity of oleanolic acid trisaccharide saponins. Carbohydrate Research, 2017, 442:9-16.
[0028] Dissolve L-arabinose, L-rhamnose (L-rhamnose, L-Rha) and D-glucose (D-glucose, D-Glc) in pyridine respectively, add benzoyl chloride dropwise under ice bath, and then rise to The whole benzoyl-protected monosaccharide can be obtained after overnight reaction at room temperature. 1-bromoglycosyl donor compounds (10a, 10b, 10c) can be obtained by reacting perbenzoyl sugar with 33% hydrobromic acid (acetic acid solution) at room temperature.
[0029] (S1) 3-O-α-L-rhamnopyranosyl (1→2)-α-L-arabinopyranosyl oleanolic acid
[0030] The compound 3-O-[2″,3″,4″-tribenzoyl-α-L-rhamnosyl (1→2)-3′,4′-diacetyl]-α-L- Arabinosyl oleanolic acid (18) (200mg, 0.181mmol) dissolved in CH 2 Cl 2...
Embodiment 2
[0037] Embodiment 2: Separation of monomeric compound in Huang Hua Pai Jiang
[0038] Prepared with reference to the method reported in the literature of the present invention: Gao Liang, Research on the chemical constituents of Huanghua Patitima, Master Thesis of Soochow University, 2011.
[0039]From Huanghua Paijiang, the following compounds P1~P9 can be isolated and obtained: 3-O-β-D-xylopyranose (1→3)-α-L-rhamnopyranose (1→2)-β- D-xylopyranose oleanolic acid 28-O-β-D-glucopyranose (P1); 3-O-β-D-glucopyranose (1→4)-β-D-glucopyranose Xylose (1→3)-α-L-rhamnopyranose (1→2)-β-D-xylopyranose oleanolic acid 28-β-D-glucopyranose side (P2); 3-O-α-L-rhamnopyranose (1→2)-β-D-xylopyranose oleanolic acid 28-O-β-D-glucopyranose side (P3); 3- O-β-D-xylopyranose(1→3)-α-L-rhamnopyranose(1→2)-α-L-arabinopyranose oleanolic acid 28-O-β-D -Glucopyranoside (P4); 3-O-β-D-xylopyranose(1→3)-α-L-rhamnopyranose(1→2)-β-D-xylopyranose Sugar oleanolic acid 28-O-β-D-glucopyranosyl-(1→6)-β-D-glucopy...
experiment example 3
[0040] Experimental Example 3: Investigation of the anti-inflammatory effect of the compound
[0041] Take the patronarin compound in Example 2 for testing. Take 20-25g mice (10 mice / group), and randomly divide them into five groups: P1-P9 group (100mg / kg), oleanolic acid control group (100mg / kg) and model group. Two hours after the last dosing, apply 25 microliters of xylene evenly to the right ear of the mouse, kill the mouse half an hour later, use a 6mm diameter puncher, take the same parts of the left and right ears of the mouse, and weigh them with an analytical balance . The weight of the removed right ear minus the weight of the left ear was the degree of swelling, the mean and standard deviation of the control group and the treatment group were calculated, and the t test was used to compare the significance of the differences between the groups. Calculate the swelling inhibition rate according to the following formula:
[0042] Swelling inhibition rate (%)=[1-avera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com