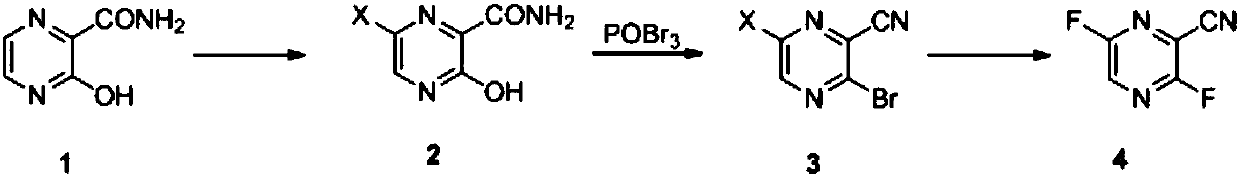Favipiravir intermediate and synthetic method for favipiravir
A compound and reaction technology, applied in the field of organic chemical synthesis, can solve the problems of unsuitability for industrial production, harsh reaction conditions, cumbersome post-processing, etc., and achieve the effects of stable yield, increased yield, and simplified post-processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0059] Preparation Example 1: Synthesis of 3,6-difluoro-2-cyanopyrazine
[0060] Step a): Under nitrogen protection, 35 g of 3-hydroxy-2-pyrazinamide, 33.6 g of pyridine and 0.3 liter of acetonitrile were mixed and heated to 80°C-85°C. 40 g of NCS was added dropwise in batches while keeping the temperature of the reaction system at 80°C to 85°C. After the addition was complete, the reaction was maintained at this temperature for 4 hours. Subsequently, the solvent was recovered by concentration. Then 0.6 liter of water was added, the resulting suspension was stirred at 20° C. to 25° C. for 2 hours, and filtered to obtain a wet product of 6-chloro-3-hydroxy-2-pyrazinamide. The wet product was vacuum-dried at 50°C-55°C to obtain 38.8 g of off-white solid 6-chloro-3-hydroxy-2-pyrazinamide. The yield was 88.7%, and the purity determined by HPLC was 99.1%.
[0061] Step b): Mix 36.7 g of 6-chloro-3-hydroxy-2-pyrazinamide and 0.3 liter of chlorobenzene, and cool to 0°C to 5°C. A...
preparation example 2
[0065] Preparation Example 2: Synthesis of 3,6-difluoro-2-cyanopyrazine
[0066] Step a): Under the protection of nitrogen, take 175 grams of 3-hydroxy-2-pyrazinamide, 168 grams of pyridine and 1.7 liters of N,N-dimethylformamide, mix and heat to 80 ° C ~ 85 ° C, and then dropwise add 290 gram of bromine while keeping the temperature of the reaction system at 80°C to 85°C. After the addition was complete, the reaction was maintained at this temperature for 2 hours. Subsequently, the solvent was recovered by concentration. Then 1.5 liters of water was added, the resulting suspension was stirred at 20° C. to 25° C. for 2 hours, and filtered to obtain a wet product of 6-bromo-3-hydroxy-2-pyrazinamide. The wet product was vacuum-dried at 50°C-55°C to obtain 220 g of off-white solid 6-bromo-3-hydroxy-2-pyrazinamide. The yield was 80.1%, and the purity determined by HPLC was 100%.
[0067] Step b): Mix 220 g of 6-bromo-3-hydroxy-2-pyrazinamide and 1.8 liters of acetonitrile, and...
preparation example 3
[0071] Preparation Example 3: Synthesis of Favipiravir
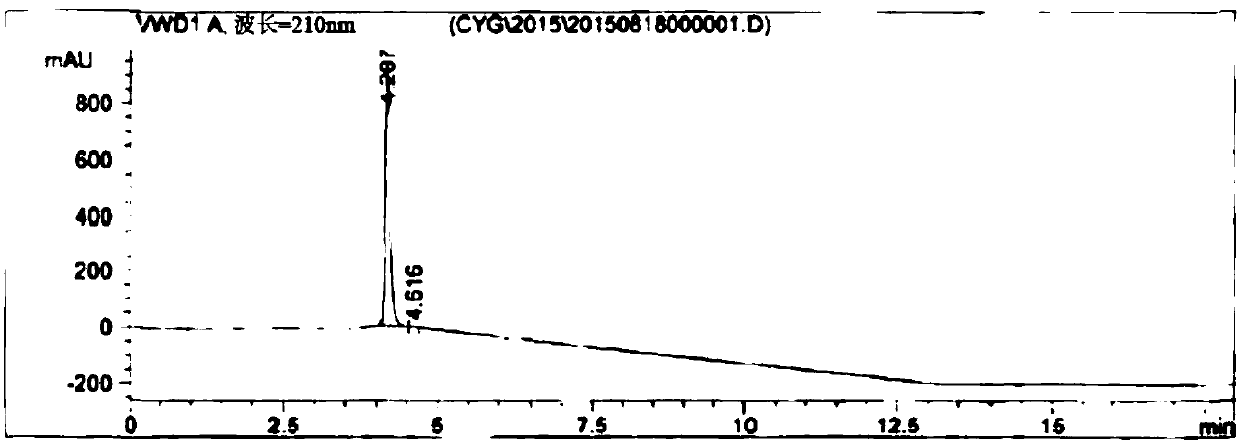
[0072] Mix 39.9 g of 3,6-difluoro-2-cyanopyrazine and 72 g of anhydrous sodium acetate in 0.35 liter of water and 0.35 liter of tetrahydrofuran, and heat to reflux for 20 hours. After the reaction is complete, the reaction solution is concentrated, and then the pH value of the system is adjusted to 2.0 to 2.5 with 20% dilute sulfuric acid. Then it was extracted with 0.2 liter of ethyl acetate. The resulting organic phase was concentrated under reduced pressure to obtain an oil. Add 0.2 liter of acetone and 50 g of dicyclohexylamine, stir the resulting suspension at 0° C. to 5° C. for 2 hours, and filter to obtain a wet product of 6-fluoro-3-hydroxy-2-cyanopyrazine dicyclohexylamine salt. Then vacuum-dried at 50° C. for 3 hours to obtain 62.4 g of a light brown solid with a yield of 70.3% and a purity of 99.2% as determined by HPLC.
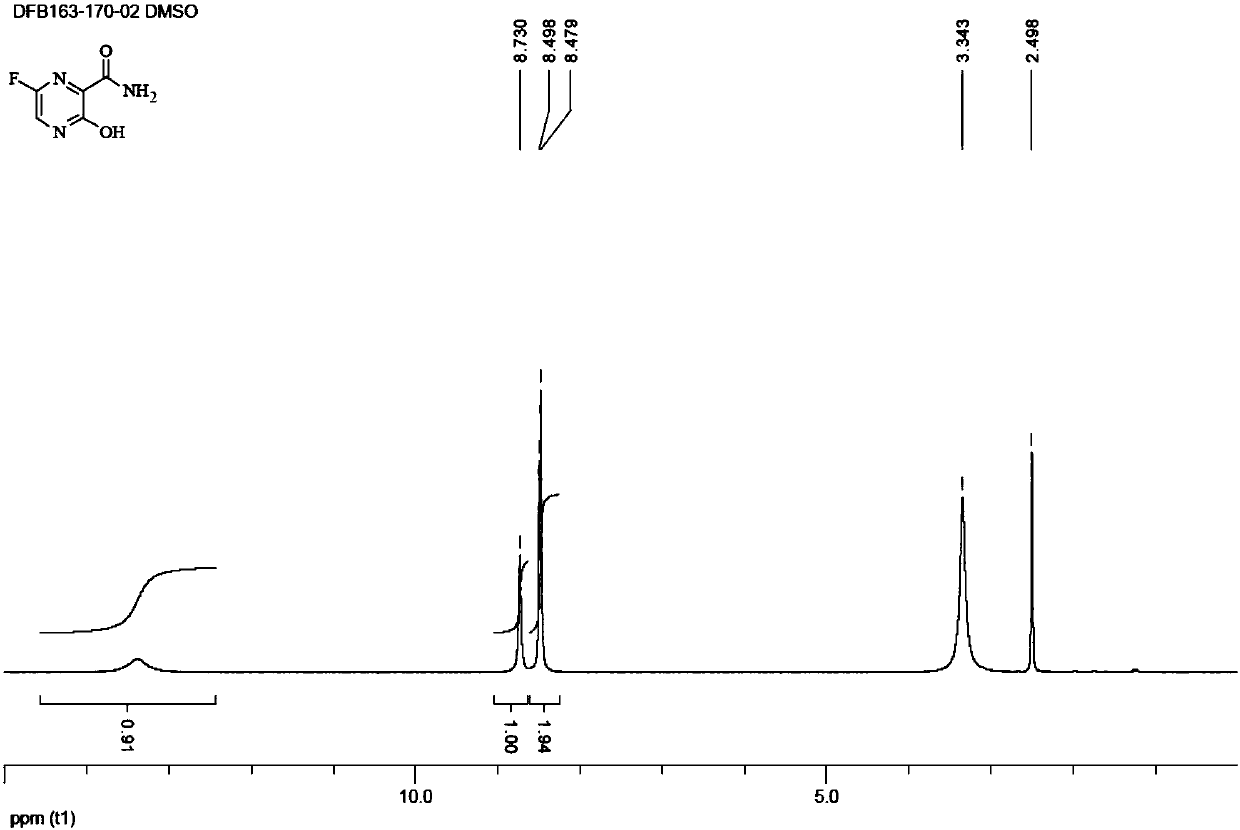
[0073] 1 H NMR(DMSO)δ1.06(m,2H),1.23(m,8H),1.61(m,2H),1.73(m,4H),1.98(m,4H),3.08(m,2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com