A kind of fused ring non-fullerene acceptor material and its preparation method and application
A non-fullerene acceptor and fused ring technology, applied in the field of electrochemical materials, can solve problems such as limiting the light absorption capacity of materials, achieve high electron mobility, high short-circuit current and energy conversion efficiency, and increase the effect of electron cloud density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
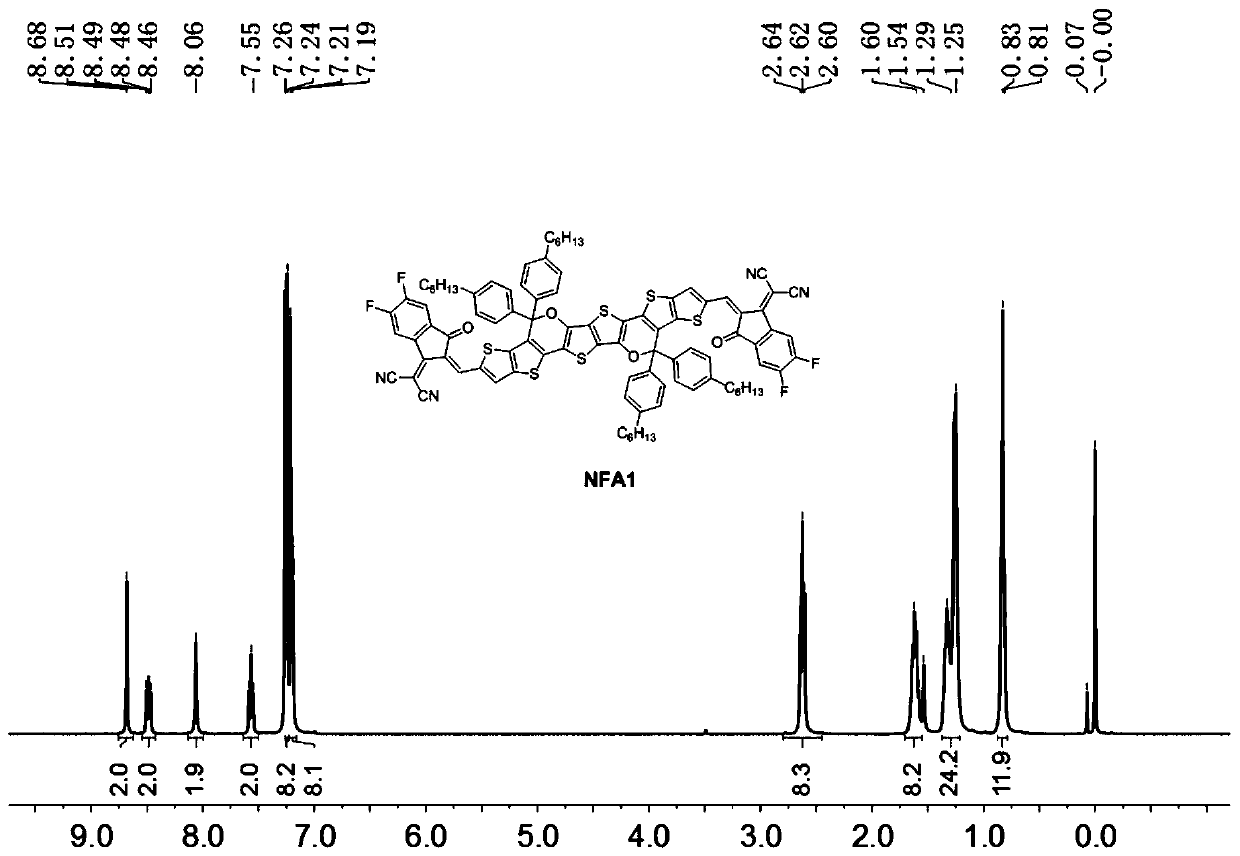
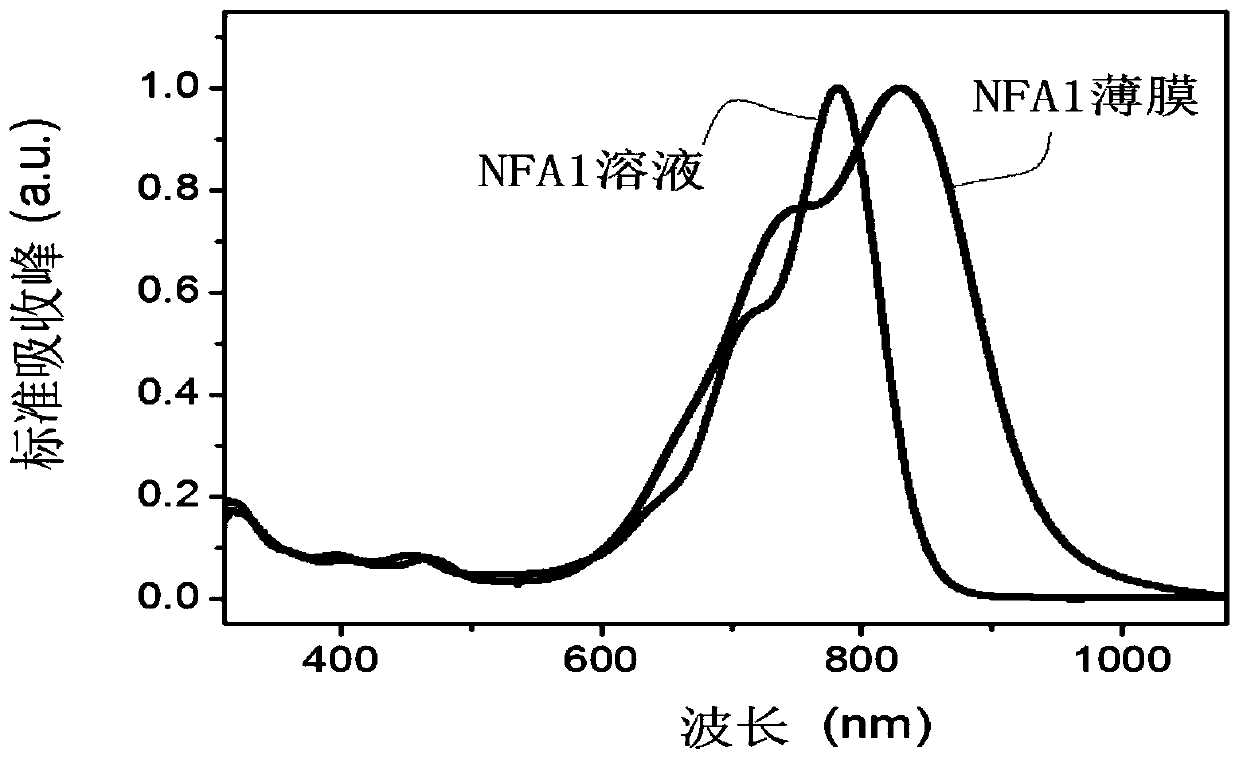
[0109] In this example, a fused-ring non-fullerene acceptor material as shown in the structure of formula I is prepared by the following steps, named NFA1, wherein R is Ar 1 for Ar 2 for The donor of the EG group is Specific steps are as follows:
[0110] (1) Compound a1 and compound b1 are coupled under catalyst catalysis to generate compound c1:
[0111]
[0112] In a 100mL Schlenck tube, sequentially add brominated raw material a1 (950 mg), tin-substituted raw material b1 (746 mg), Pd (PPh 3 ) 4 (164mg), toluene (50mL), DMF (3.8mL), heated to reflux under nitrogen protection for 48 hours, removed the toluene by rotary evaporation under reduced pressure and passed through a silica gel column with chloroform as the eluent to obtain a yellow solid product c1 (774mg, produced rate 89%).
[0113] NMR and mass spectrometry data of c1: 1 H NMR (CDCl 3 ,400MHz,δ / ppm):7.49(d,J=5.3Hz,2H),7.26(d,J=5.3Hz,2H),4.38(q,J=7.1Hz,4H),4.02(s,6H) ,1.35(t,J=7.1Hz,6H). 13 C NMR...
Embodiment 2
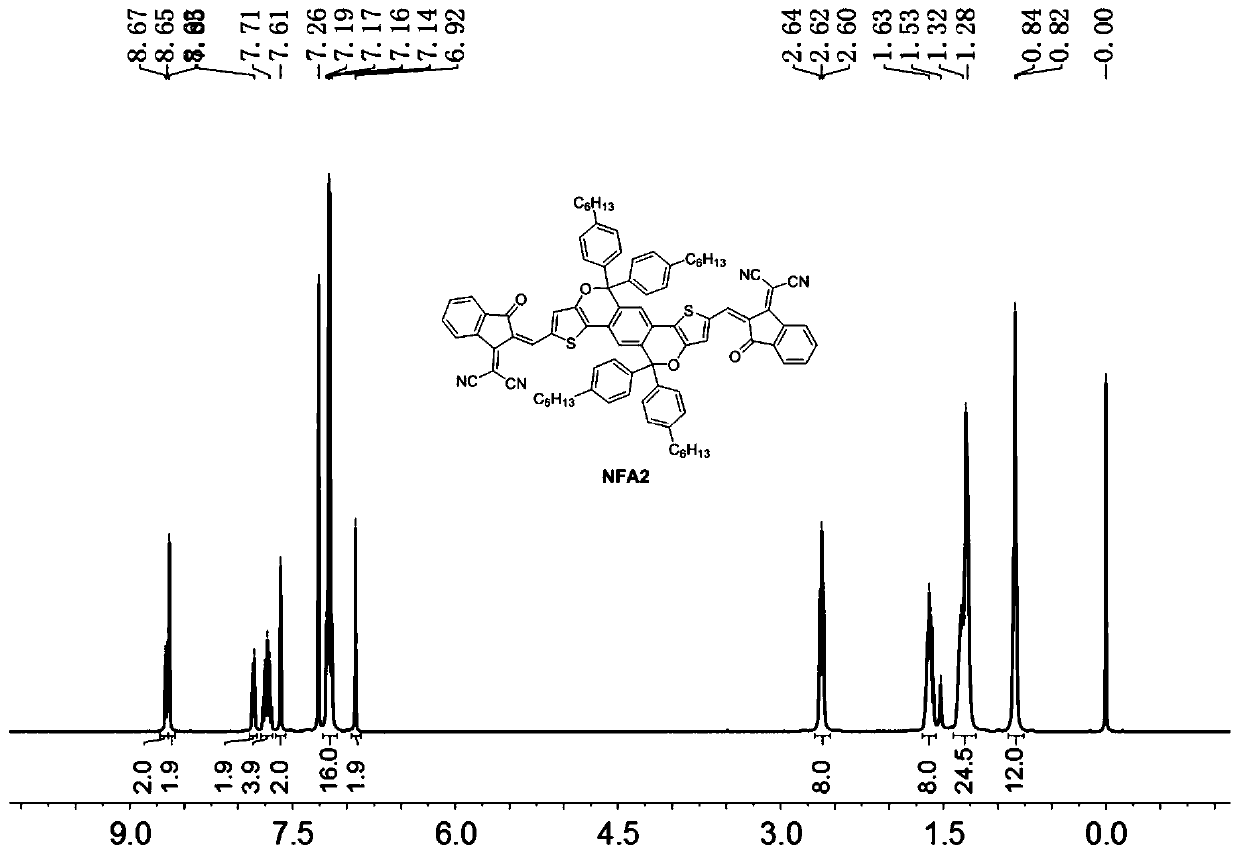
[0135] In this example, a fused-ring non-fullerene acceptor material as shown in the formula II structure is prepared by the following steps, named NFA2, wherein R is Ar 1 for Ar 2 for The donor of the EG group is Specific steps are as follows:
[0136] (a) Compound a1' and compound b1' are coupled under catalyst catalysis to generate compound c1':
[0137]
[0138] Add tin-substituted raw material a1' (4.7g), brominated raw material b1' (2.8g), Pd(PPh 3 ) 4 (890mg), toluene (50mL), heated to reflux under nitrogen protection for 40 hours, removed the toluene by rotary evaporation under reduced pressure, and passed through a silica gel column with dichloromethane as the eluent to obtain the yellow solid product c1' (2.5g, yield 74 %). Mass spectral data of c1': MALDI-TOFMS (m / z): 446.2 (M + ).
[0139] (b) Compound c1' reacts with boron tribromide at room temperature to generate compound d1':
[0140]
[0141] In a 100mL Schlenck tube, add c1' (446mg), dry ...
Embodiment 3
[0159] In this example, a fused ring non-fullerene acceptor material with the structure shown in formula I was prepared by the following steps, named NFA3, wherein R is C 8 straight chain alkyl, Ar 1 for Ar 2 for The donor of the EG group is Specific steps are as follows:
[0160] (1) Compound a2 and compound b2 are coupled under catalyst catalysis to generate compound c2:
[0161]
[0162] Into a 100mL Schlenck tube, add brominated raw material a2 (330mg), tin-substituted raw material b2 (212mg), Pd(PPh 3 ) 4 (50mg), toluene (15mL), DMF (1mL), heated to reflux under nitrogen protection for 60 hours, decompressed and rotary evaporated to remove toluene, and passed through a silica gel column, with chloroform as eluent, to obtain yellow solid product c2 (219mg, yield 71%). Mass spectrometry data of c2: MALDI-TOF MS(m / z): 671.0(M + ).
[0163] (2) Compound c2 reacts with boron tribromide at room temperature to generate compound d2:
[0164]
[0165] In a 100m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron mobility | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com