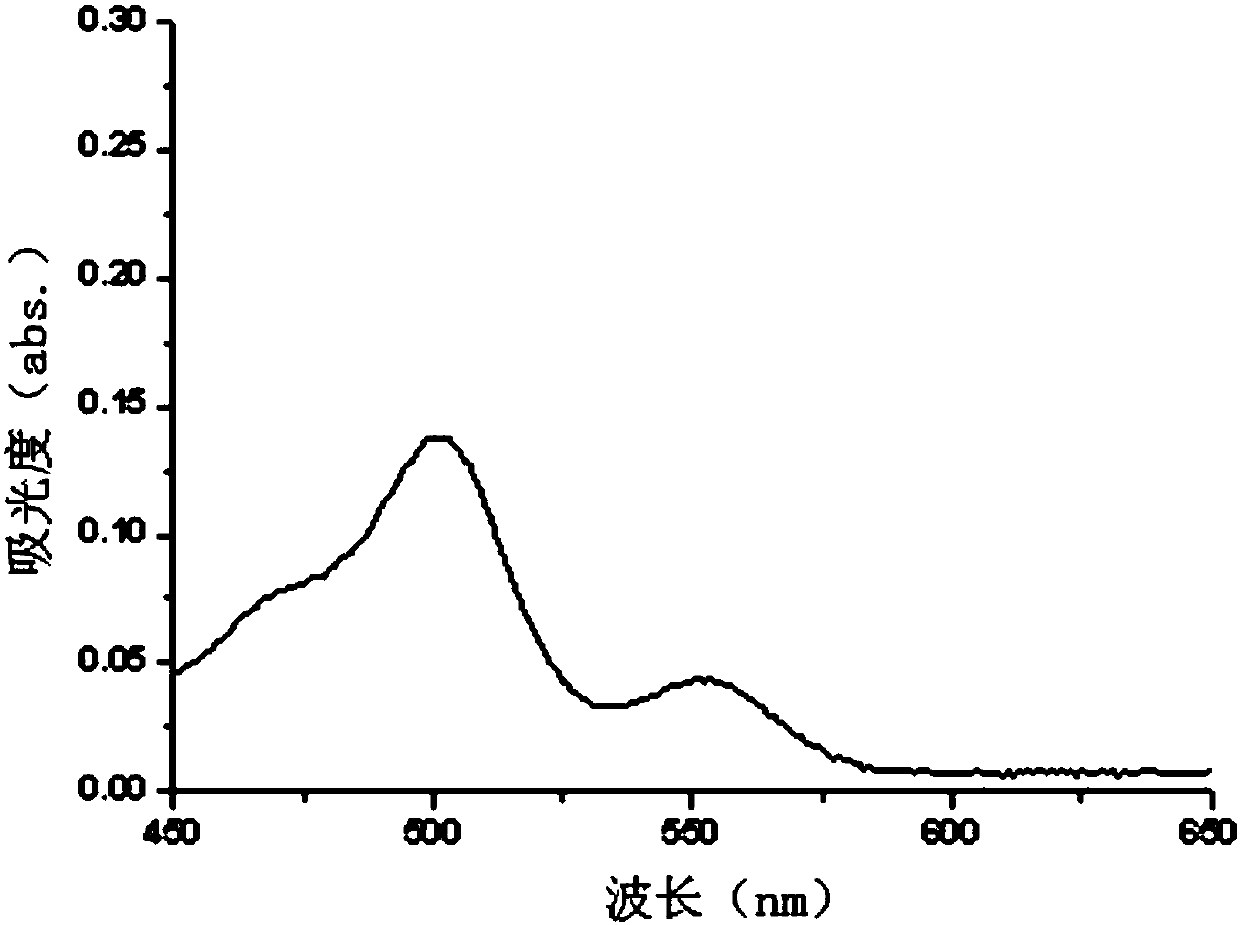
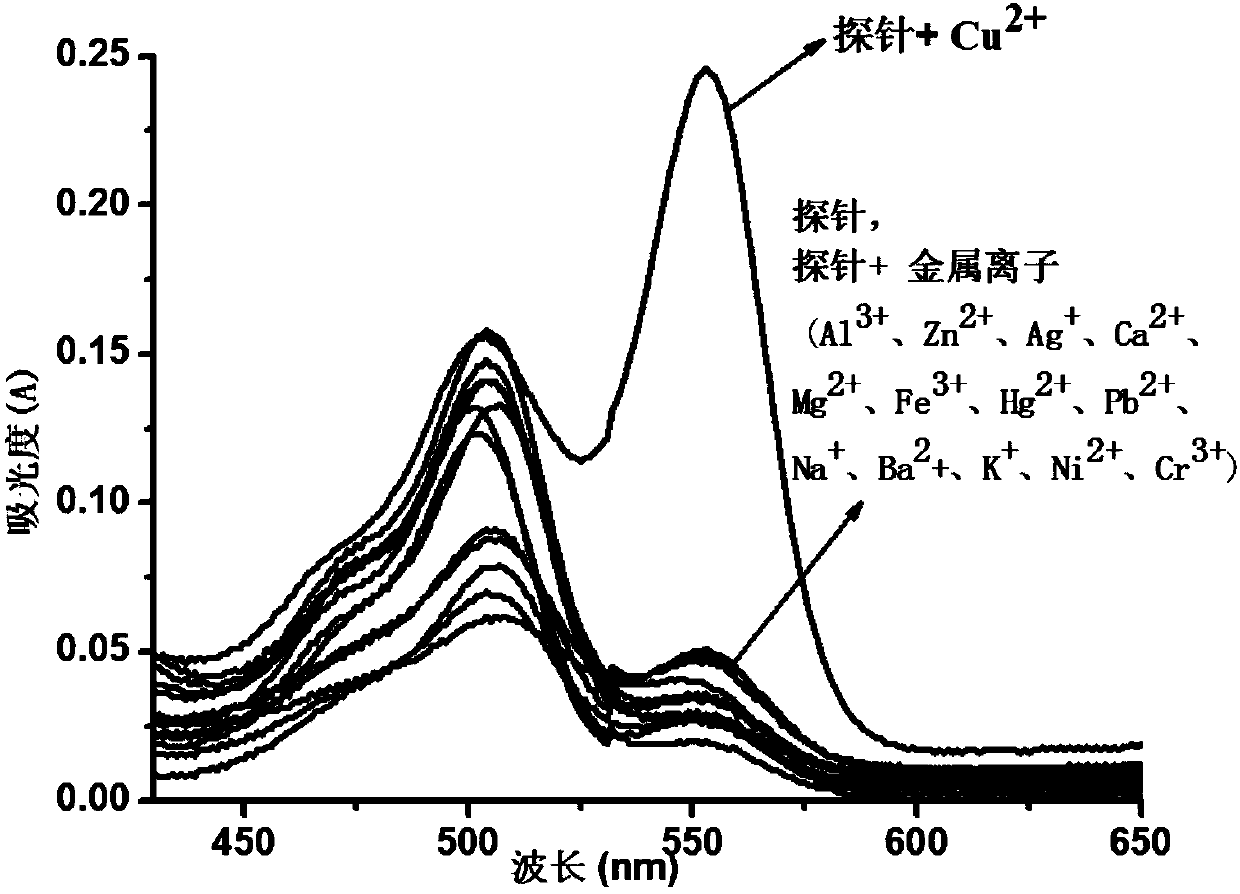
Fluorescein-rhodamine B copper ion ultraviolet molecular probe applied to naked eye detection and synthesizing and using methods thereof
A molecular probe and fluorescein technology, which is applied to the fluorescein-rhodamine B copper ion ultraviolet molecular probe for naked eye detection and the fields of its synthesis and use, can solve problems such as high price, and achieve low price and high application Value, simple effect of detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0027] Specific embodiment one: the structural formula of the fluorescein-rhodamine B copper ion ultraviolet molecular probe for naked eye detection of the present embodiment is:
[0028]
specific Embodiment approach 2
[0029] Specific embodiment two: the synthetic method of the fluorescein-rhodamine B copper ion ultraviolet molecular probe that is used for naked-eye detection described in specific embodiment one is as follows:
[0030] 2. According to the molar ratio of fluorescein monoaldehyde and rhodamine B hydrazine as 1: (1 ~ 5), respectively add fluorescein monoaldehyde and rhodamine B hydrazine to alcohol solvent, heat to boiling and reflux, and react for 6 ~ 10h, after the end of the reaction, it was cooled and concentrated to obtain a reaction mixture;
[0031] 2. Take a column chromatography column filled with 200-300 mesh silica gel, use the mixture of dichloromethane and methanol as the eluent, wash the mixture through column chromatography gradient gradient, and separate to obtain Fluorescein-rhodamine B copper ion ultraviolet molecular probe.
specific Embodiment approach 3
[0032] Embodiment 3: The difference between this embodiment and Embodiment 2 is that the alcohol solvent in step 1 is methanol, ethanol, propanol, isopropanol or butanol; the others are the same as Embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com