Oral solid preparation and application thereof
A technology of solid preparation and disintegrant, which is applied in the field of pharmaceutical preparations to achieve the effects of improving dissolution rate and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Embodiment 1: the preparation of formula (A) compound
[0108] Dissolve the compound of formula (B) (1.0g) in dichloromethane (5ml), stir at room temperature to form a solution, add potassium phthalimide (0.27g) to the solution, keep the reaction for 4h, cool to -50 °C, filtered, and the solvent was spin-dried to obtain a solid that was the compound of formula (A) (amorphous).
[0109] Melting point: 135-145°C.
[0110] MS / HRMS m / z: 717[M+H] + ;677[M-K] - .
[0111] 1 H-NMR (400MHz, DMSO-d 6 )δ: 1.44(t, 3H), 1.46(t, 3H), 2.38(s, 3H), 2.41(s, 3H), 2.44(s, 3H), 4.64(q, 2H), 5.29(d, 1H ), 5.32(d, 1H), 5.52(d, 1H), 5.56(d, 1H), 6.86(q, 1H), 6.90(d, 2H), 7.18(m, 2H), 7.22(d, 2H) , 7.33 (m, 1H), 7.36 (m, 1H), 7.46 (d, 1H), 7.52 (dd, 1H), 7.75 (d, 1H).
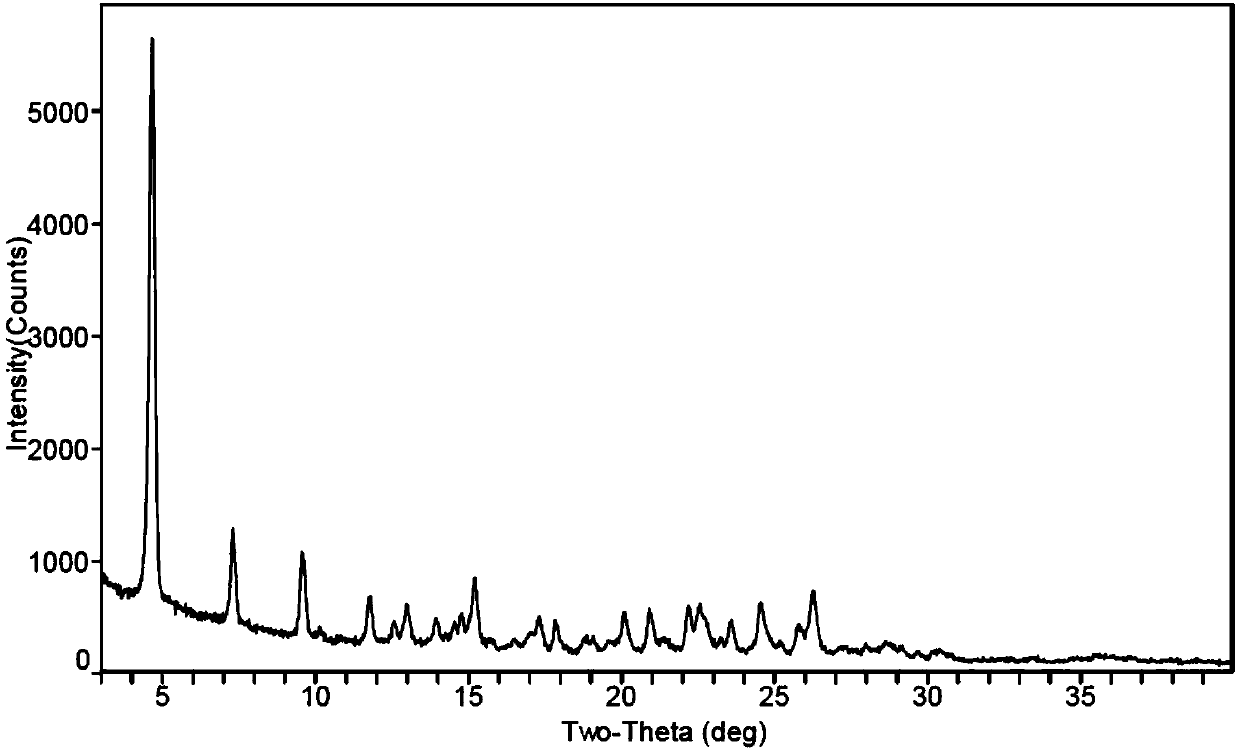
[0112] 1 H-NMR spectrum and X-ray powder diffraction spectrum are shown in Figure 5 and Figure 6 .
Embodiment 2
[0113] Embodiment 2: Antihypertensive efficacy test of formula (A) compound on spontaneously hypertensive rats
[0114] Spontaneously hypertensive rats aged 12 weeks (hereinafter referred to as SHR, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.) were anesthetized by intraperitoneal injection of 2.5% sodium pentobarbital. The blood pressure sensing catheter was inserted into the abdominal aorta, the implant was fixed on the abdominal wall, and the postoperative daily care was performed after suturing. Animals whose systolic blood pressure exceeded 160mm Hg were selected into groups, with 8 animals in each group, 3 groups in total. The matched group is given 0.5% sodium carboxymethylcellulose (hereinafter referred to as CMC-Na); formula (B) compound group and formula (A) compound group adopt 0.5% CMC-Na to dissolve, and dosage is all at 1mg / kg A Zisartan effective dose meter, the administration volume is 4mL / kg, all are administered by intragastr...
Embodiment 3
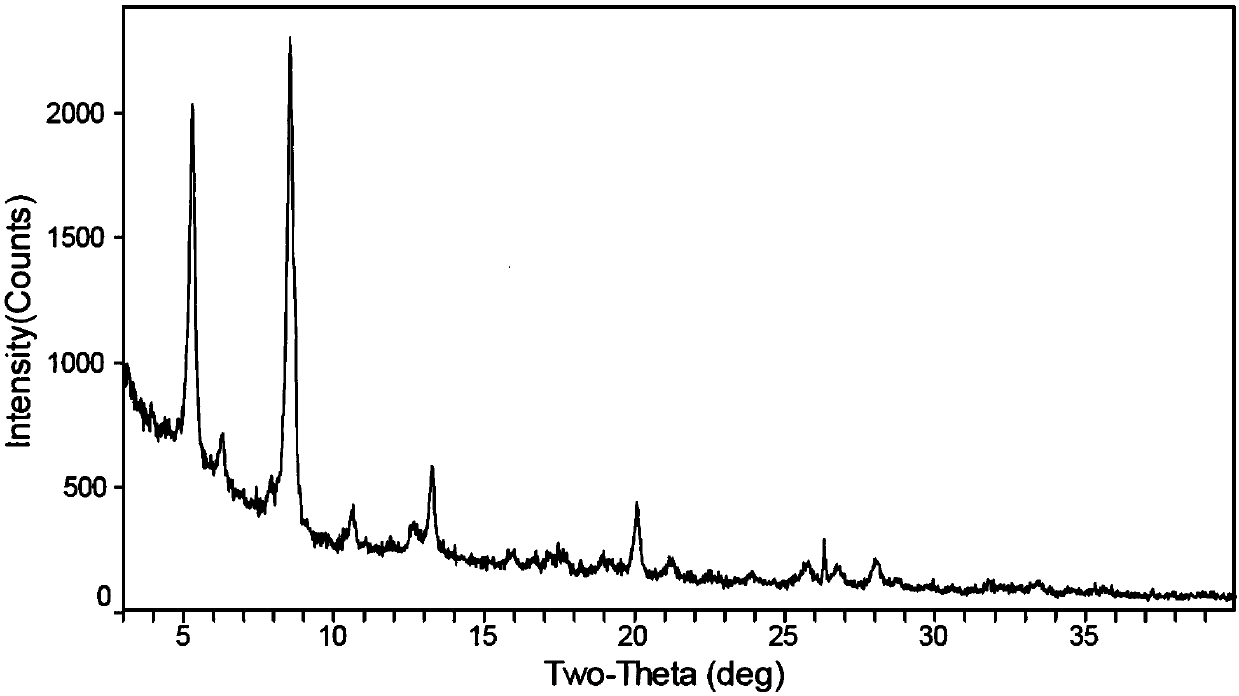
[0125] Embodiment 3: the preparation of crystal form I
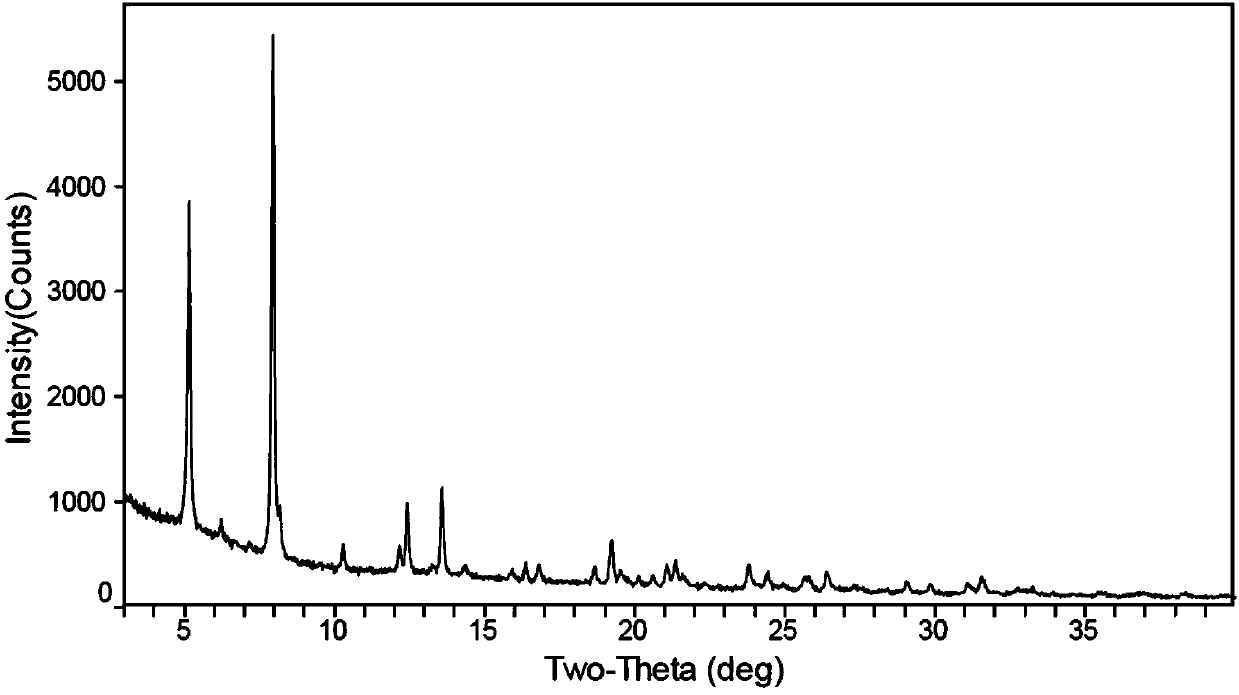
[0126] (1) Take 15 mg of the compound of formula (A), add 0.2 ml of ethanol / isopropyl ether (volume ratio 1:5) mixed solution to obtain a suspension, stir at room temperature for 1 day, filter, and dry to obtain crystal form I. See the attached XRD detection pattern figure 1 ; DSC: 184°C. According to the same method, use ethanol / n-heptane (volume ratio of 1:5) mixed solution, isopropanol / n-heptane (volume ratio of 1:5) mixed solution, or tetrahydrofuran / n-heptane (volume ratio of 1:5) mixed solution was also prepared to obtain the crystal form I.
[0127] (2) Dissolve 15 mg of the compound of formula (A) in 0.1 ml of methanol to obtain a clear solution, add 1.0 ml of isopropyl ether under stirring, and precipitate a solid, continue stirring, filter, and dry to obtain crystal form I. In the same way, use good solvent ethanol / antisolvent isopropyl ether, good solvent ethanol / antisolvent methyl tert-butyl ether, good so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com