Preparation methods of niraparib and intermediate of niraparib as well as intermediate compound
A technology for compounds and intermediates, which is applied to the preparation of niraparib and its intermediates and the field of intermediate compounds, can solve the problems of high production cost, cumbersome enzyme catalysis operations, expensive purchase price of enzymes, etc., and achieves low cost and high productivity. The effect of high rate and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
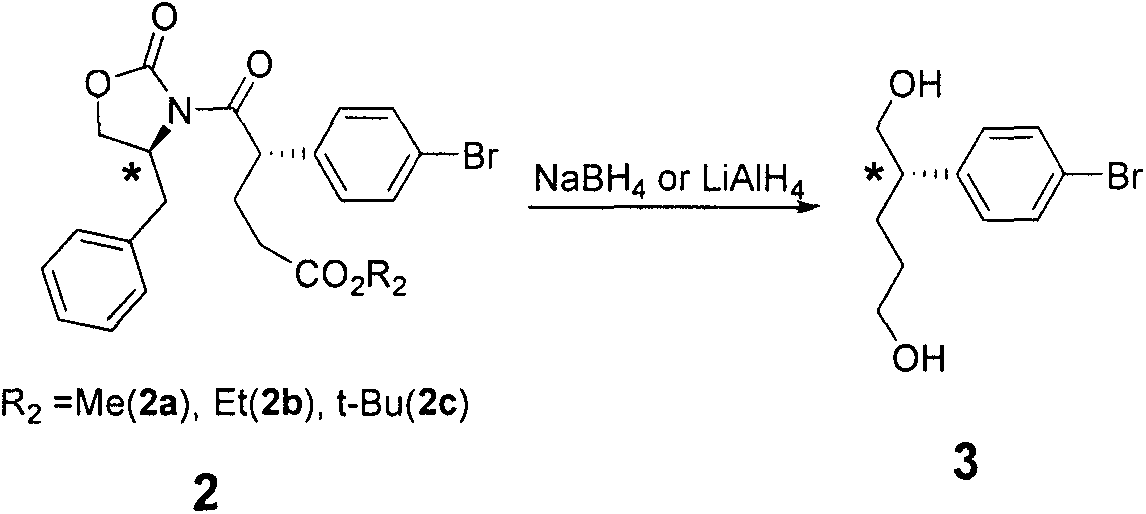
Embodiment 1
[0047] Synthesis of Example 1 Compound 1 (1a, 1b, 1c)
[0048]
[0049] Synthesis of 1a: In a 1000mL eggplant-shaped bottle replaced with nitrogen, 86g (0.4mol) of p-bromophenylacetic acid, 35.4g (0.2mol) of (S)-4-benzyl-2-oxazolidinone, and 112ml of triethylamine ( 0.8mol) and 350mL toluene, stirred and heated to 80°C, dissolved 49.5ml (0.4mol) of pivaloyl chloride in 300ml toluene, slowly dropped into the reaction solution, after the addition was completed, heated to 110°C, reacted for 14h, TLC showed the reaction completely, cooled to room temperature, filtered, the filter cake was washed twice with ethyl acetate, and the organic phases were combined. The organic phase was washed with water, washed with dilute hydrochloric acid (2M), washed with 5% sodium bicarbonate, dried over anhydrous sodium sulfate, and mixed. Filtration, concentration under reduced pressure, separation by silica gel column chromatography to obtain 62.1 g of white solid (1a), yield 83%, purity 98%,...
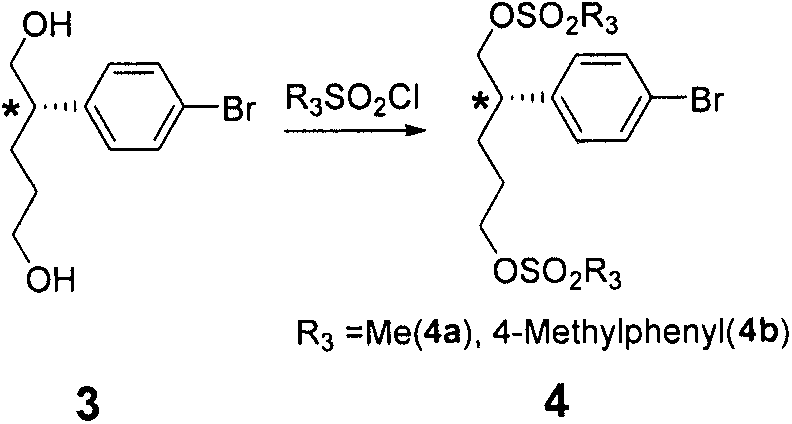
Embodiment 2
[0052] Synthesis of Example 2 Compound 2 (2a, 2b, 2c, 2d, 2e)
[0053]
[0054] Synthesis of 2a
[0055] Method 1: In a 2L three-neck bottle replaced with nitrogen, add 14.3ml (0.128mol) of titanium tetrachloride and 450mL of dichloromethane, cool to 0 degrees, slowly add 12ml (0.04mol) of tetraisopropyl titanate dropwise, and drop After stirring at 0°C for 15 minutes, 31 ml of diisopropylethylamine (0.176) was added dropwise. After dripping, stir for 30 minutes. 60 g (0.16 mol) of compound (1a) was dissolved in 400 ml of dichloromethane and added dropwise to the above reaction solution, and stirred for 2 h after the drop was completed. Then 43ml (0.48mol) of methyl acrylate was added dropwise, and stirred at 0°C for 3 days. After the reaction was complete, 1 L of saturated ammonium chloride solution and 150 g of diatomaceous earth were added, filtered, and the filter cake was washed twice with dichloromethane. The dichloromethane layer was washed with 1M dilute hydroch...
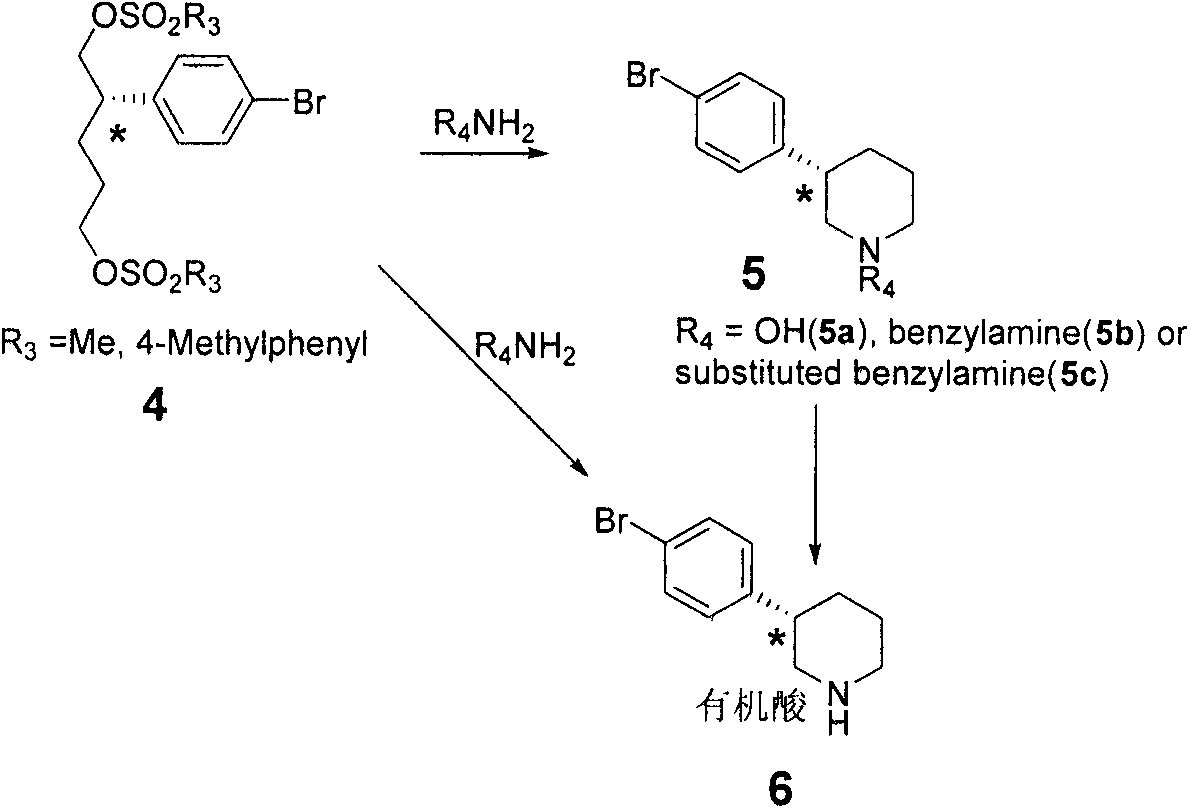
Embodiment 3
[0061] The synthesis of embodiment 3 compound 3
[0062]
[0063]Sodium borohydride reduction method: In a 1L eggplant-shaped bottle, add 50g (0.108mmol) or (2b) of compound (2a), 250ml methanol and 250ml tetrahydrofuran, cool to 0 degrees, add sodium borohydride 16.8g (0.434mmol ). React at 0°C for 3h, slowly rise to room temperature, and stir overnight. TLC showed that the reaction was complete, cooled to 0°C, quenched by adding 2M dilute hydrochloric acid, removed methanol and tetrahydrofuran under reduced pressure, extracted with ethyl acetate (3×300mL), combined organic layers, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, silica gel Column chromatography separation gave white solid (3) 22.6g, yield 81%, purity 98.3%, 1H NMR (500MHz, Chloroform-d) δ7.50-7.36 (m, 2H), 7.16-7.05 (m, 2H) , 3.79-3.64(m, 2H), 3.58(t, J=6.4Hz, 2H), 2.76(ddt, J=11.4, 9.5, 5.7Hz, 1H), 1.83(dddd, J=13.4, 10.1, 6.2, 5.2Hz, 1H), 1.76-1.68(m, 2H), 1.59(dtd,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com