Aspirin-DL-lysine freeze-dried powder injection for injection and preparation method thereof
A technology of freeze-dried powder injection and lysine-pirin, which is applied in the field of lysine-pirin freeze-dried powder for injection and its preparation, can solve problems such as increased irritation and sensitization, muscle necrosis, and allergic reactions, and achieve Improved stability, stable drug properties, and good formability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
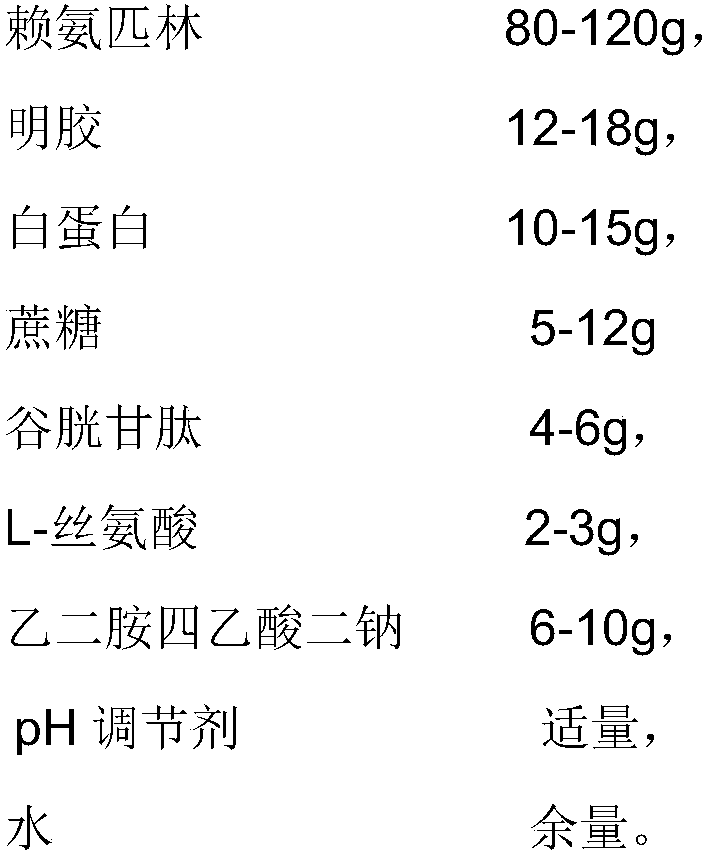
[0029] 1. Prescription
[0030]
[0031] 2. Production process
[0032] 2.1 Control the cleaning and sterilization of antibiotic glass bottles
[0033] Controlled antibiotic glass bottles are removed from the outer packaging, passed into the bottle washing and drying room, cleaned by a bottle washing machine, dried with compressed air, dried and sterilized in a hot air tunnel oven at 350°C for 5 minutes, and cooled.
[0034] 2.2 Cleaning and sterilization of butyl rubber
[0035] The butyl rubber stoppers are removed from the outer packaging and sent to the stopper washing room, cleaned by a stopper washing machine, sterilized by damp heat at 121°C for 40 minutes, and cooled for later use.
[0036] 2.3 Sterilization of aluminum-plastic combination caps
[0037] The aluminum-plastic cover is removed from the outer packaging, and then transferred to the aluminum cover sterilization room, sterilized by dry heat at 110°C for 120 minutes in a drying oven, cooled, and set asid...
Embodiment 2
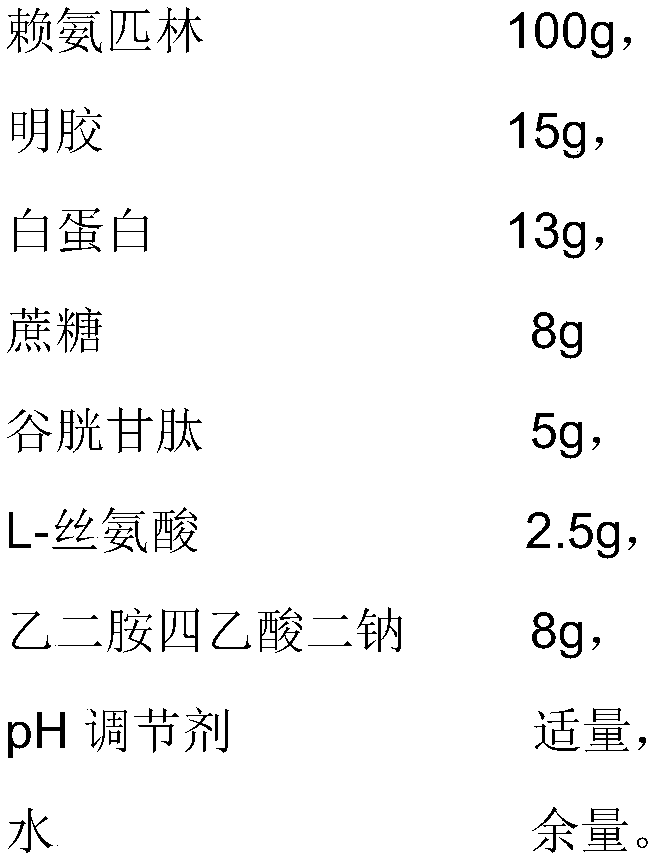
[0049] The difference between the present embodiment and embodiment 1 is that the prescription is different, and the prescription of the present embodiment is as follows:
[0050]
Embodiment 3
[0052] The difference between the present embodiment and embodiment 1 is that the prescription is different, and the prescription of the present embodiment is as follows:
[0053]
[0054]
[0055] Stability study
[0056] Get the lysine-pirin freeze-dried powder sample that the embodiment of the present invention 1-3 makes and remove outer packaging, place 10 days in room temperature air, take samples respectively in the 1st, 3rd, 5th, 10th days after setting out, investigate relevant Project, all sample properties have no change, it is white crystalline powder, pH value is stable, is 5.6. Clarity, free salicylic acid content, and sterility test all meet the standard (((National Drug Standard)) WS1-XG-015-2001Z, the same below), and the content is greater than 99.2%.
[0057] Take the lysine-pirin freeze-dried powder samples prepared in Examples 1-3 of the present invention, place them at room temperature, and take samples 1, 3, 6, and 11 months after setting out, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com